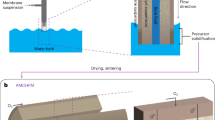
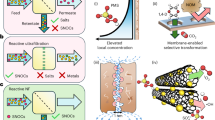
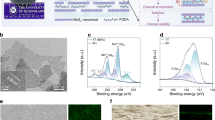
Abstract
Many chemical transformations that are desirable at large scales either lack economically or environmentally sustainable catalytic solutions or require significant improvement. In many cases, identifying catalytic active centers that can perform these reactions under desired conditions and at acceptable rates, stabilities and selectivity has been difficult. One possible approach to overcome this issue is to design membrane–catalyst systems that can increase catalytic rates or selectivity. In general, this membrane–catalyst concept has been challenging to implement, optimize and even thoroughly study as the development of membranes and catalysts is usually undertaken in different scientific communities. Approaches where these two building blocks are co-optimized have not been rigorously explored. In this Perspective, strategies for integrating membrane and catalyst functionalities at molecular scales is explored, with the aim to develop reactive systems for difficult chemical transformations. Specifically, the challenges and opportunities of membrane–catalyst systems for oxidative and non-oxidative shale-gas conversion chemistries are critically examined.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Xin, H. & Linic, S. Analyzing relationships between surface perturbations and local chemical reactivity of metal sites: alkali promotion of O2 dissociation on Ag(111). J. Chem. Phys. 144, 234704 (2016).
Xin, H., Holewinski, A. & Linic, S. Predictive structure–reactivity models for rapid screening of Pt-based multimetallic electrocatalysts for the oxygen reduction reaction. ACS Catal. 2, 12–16 (2012).
Schwach, P., Pan, X. & Bao, X. Direct conversion of methane to value-added chemicals over heterogeneous catalysts: challenges and prospects. Chem. Rev. 117, 8497–8520 (2017).
McFarland, E. Unconventional chemistry for unconventional natural gas. Science 338, 340–342 (2012).
Wortman, J., Igenegbai, V. O., Almallahi, R., Motagamwala, A. H. & Linic, S. Optimizing hierarchical membrane/catalyst systems for oxidative coupling of methane using additive manufacturing. Nat. Mater. 22, 1523–1530 (2023).
Almallahi, R., Wortman, J. & Linic, S. Overcoming limitations in propane dehydrogenation by codesigning catalyst-membrane systems. Science 383, 1325–1331 (2024).
Farrell, B. L., Igenegbai, V. O. & Linic, S. A viewpoint on direct methane conversion to ethane and ethylene using oxidative coupling on solid catalysts. ACS Catal. 6, 4340–4346 (2016).
Igenegbai, V. O., Almallahi, R., Meyer, R. J. & Linic, S. Oxidative coupling of methane over hybrid membrane/catalyst active centers: chemical requirements for prolonged lifetime. ACS Energy Lett. 4, 1465–1470 (2019).
Xu, S. J. & Thomson, W. J. Perovskite-type oxide membranes for the oxidative coupling of methane. AlChE J. 43, 2731–2740 (1997).
Dimitrakopoulos, G., Koo, B., Yildiz, B. & Ghoniem, A. F. Highly durable C2 hydrocarbon production via the oxidative coupling of methane using a BaFe0.9Zr0.1O3−δ mixed ionic and electronic conducting membrane and La2O3 catalyst. ACS Catal. 11, 3638–3661 (2021).
Czuprat, O., Schiestel, T., Voss, H. & Caro, J. Oxidative coupling of methane in a BCFZ perovskite hollow fiber membrane reactor. Ind. Eng. Chem. Res. 49, 10230–10236 (2010).
Othman, N. H., Wu, Z. & Li, K. An oxygen permeable membrane microreactor with an in-situ deposited Bi1.5Y0.3Sm0.2O3−δ catalyst for oxidative coupling of methane. J. Membr. Sci. 488, 182–193 (2015).
Garcia-Fayos, J., Lobera, M. P., Balaguer, M. & Serra, J. M. Catalyst screening for oxidative coupling of methane integrated in membrane reactors. Front. Mater. 5, 31 (2018).
Wang, H., Cong, Y. & Yang, W. High selectivity of oxidative dehydrogenation of ethane to ethylene in an oxygen permeable membrane reactor. Chem. Commun. 2002, 1468–1469 (2002).
García-Fayos, J., Ruhl, R., Navarrete, L., Bouwmeester, H. J. & Serra, J. M. Enhancing oxygen permeation through Fe2NiO4–Ce0.8Tb0.2O2−δ composite membranes using porous layers activated with Pr6O11 nanoparticles. J. Mater. Chem. A 6, 1201–1209 (2018).
Serra, J. M., Garcia-Fayos, J., Baumann, S., Schulze-Küppers, F. & Meulenberg, W. Oxygen permeation through tape-cast asymmetric all-La0.6Sr0.4Co0.2Fe0.8O3−δ membranes. J. Membr. Sci. 447, 297–305 (2013).
Baumann, S. et al. Ultrahigh oxygen permeation flux through supported Ba0.5Sr0.5Co0.8Fe0.2O3−δ membranes. J. Membr. Sci. 377, 198–205 (2011).
Schiestel, T. et al. Hollow fibre perovskite membranes for oxygen separation. J. Membr. Sci. 258, 1–4 (2005).
Tan, X. et al. Oxygen permeation behavior of La0.6Sr0.4Co0.8Fe0.2O3 hollow fibre membranes with highly concentrated CO2 exposure. J. Membr. Sci. 389, 216–222 (2012).
Cruellas, A. et al. Oxidative coupling of methane in membrane reactors; a techno-economic assessment. Processes 8, 274 (2020).
Li, S., Jin, W., Xu, N. & Shi, J. Synthesis and oxygen permeation properties of La0.2Sr0.8Co0.2Fe0.8O3−δ membranes. Solid State Ionics 124, 161–170 (1999).
Di Felice, L. et al. New high temperature sealing technique and permeability data for hollow fiber BSCF perovskite membranes. Chem. Eng. Process. Process Intensification 107, 206–219 (2016).
He, B., Zhang, K., Ling, Y., Xu, J. & Zhao, L. A surface modified La0.6Sr0.4Co0.2Fe0.8O3−δ ultrathin membrane for highly efficient oxygen separation. J. Membr. Sci. 464, 55–60 (2014).
Wang, Z., Kathiraser, Y., Soh, T. & Kawi, S. Ultra-high oxygen permeable BaBiCoNb hollow fiber membranes and their stability under pure CH4 atmosphere. J. Membr. Sci. 465, 151–158 (2014).
Wang, H., Cong, Y. & Yang, W. Oxidative coupling of methane in Ba0.5Sr0.5Co0.8Fe0.2O3−δ tubular membrane reactors. Catal. Today 104, 160–167 (2005).
Igenegbai, V. O., Meyer, R. J. & Linic, S. In search of membrane-catalyst materials for oxidative coupling of methane: performance and phase stability studies of gadolinium-doped barium cerate and the impact of Zr doping. Appl. Catal. B 230, 29–35 (2018).
Xu, S. J. & Thomson, W. J. Stability of La0.6Sr0.4Co0.2Fe0.8O3−δ perovskite membranes in reducing and nonreducing environments. Ind. Eng. Chem. Res. 37, 1290–1299 (1998).
Zavyalova, U., Holena, M., Schlögl, R. & Baerns, M. Statistical analysis of past catalytic data on oxidative methane coupling for new insights into the composition of high-performance catalysts. ChemCatChem 3, 1935–1947 (2011).
Kumar, G., Lau, S. L. J., Krcha, M. D. & Janik, M. J. Correlation of methane activation and oxide catalyst reducibility and its implications for oxidative coupling. ACS Catal. 6, 1812–1821 (2016).
Thyssen, V. V., Vilela, V. B., de Florio, D. Z., Ferlauto, A. S. & Fonseca, F. C. Direct conversion of methane to C2 hydrocarbons in solid-state membrane reactors at high temperatures. Chem. Rev. 122, 3966–3995 (2021).
Vamvakeros, A. et al. Real time chemical imaging of a working catalytic membrane reactor during oxidative coupling of methane. Chem. Commun. 51, 12752–12755 (2015).
Vamvakeros, A. et al. Real-time tomographic diffraction imaging of catalytic membrane reactors for the oxidative coupling of methane. Catal. Today 364, 242–255 (2021).
Backhaus-Ricoult, M. SOFC—a playground for solid state chemistry. Solid State Sci. 10, 670–688 (2008).
Wang, D. et al. Preparation of a Gd0.1Ce0.9O2−δ interlayer for intermediate-temperature solid oxide fuel cells by spray coating. J. Alloys Compd. 505, 118–124 (2010).
Fang, S., Yoo, C.-Y. & Bouwmeester, H. J. Performance and stability of niobium-substituted Ba0.5Sr0.5Co0.8Fe0.2O3−δ membranes. Solid State Ionics 195, 1–6 (2011).
Wachsman, E. Functionally gradient bilayer oxide membranes and electrolytes. Solid State Ionics 152, 657–662 (2002).
Brisotto, M. et al. High temperature stability of Ba0.5Sr0.5Co0.8Fe0.2O3−δ and La0.6Sr0.4Co1−yFeyO3−δ oxygen separation perovskite membranes. J. Eur. Ceram. Soc. 36, 1679–1690 (2016).
Zhang, Z. et al. Facile fabrication and improved carbon dioxide tolerance of a novel bilayer-structured ceramic oxygen permeating membrane. J. Membr. Sci. 472, 10–18 (2014).
Kim, J. P., Park, J. H., Magnone, E. & Lee, Y. Significant improvement of the oxygen permeation flux of tubular Ba0.5Sr0.5Co0.8Fe0.2O3−δ membranes covered by a thin La0.6Sr0.4Ti0.3Fe0.7O3−δ layer. Mater. Lett. 65, 2168–2170 (2011).
Liu, R. R. et al. Influence of water vapor on long-term performance and accelerated degradation of solid oxide fuel cell cathodes. J. Power Sources 196, 7090–7096 (2011).
Takanabe, K. & Iglesia, E. Mechanistic aspects and reaction pathways for oxidative coupling of methane on Mn/Na2WO4/SiO2 catalysts. J. Phys. Chem. C 113, 10131–10145 (2009).
Gambo, Y., Jalil, A. A., Triwahyono, S. & Abdulrasheed, A. A. Recent advances and future prospect in catalysts for oxidative coupling of methane to ethylene: a review. J. Ind. Eng. Chem. 59, 218–229 (2018).
Takanabe, K. & Iglesia, E. Rate and selectivity enhancements mediated by OH radicals in the oxidative coupling of methane catalyzed by Mn/Na2WO4/SiO2. Angew. Chem. Int. Ed. 47, 7689–7693 (2008).
Aydin, Z. et al. Effects of N2O and water on activity and selectivity in the oxidative coupling of methane over Mn–Na2WO4/SiO2: role of oxygen species. ACS Catal. 12, 1298–1309 (2022).
Zhu, C. et al. Electrochemical conversion of methane to ethylene in a solid oxide electrolyzer. Nat. Commun. 10, 1173 (2019).
Ramaiyan, K. P., Denoyer, L. H., Benavidez, A. & Garzon, F. H. Selective electrochemical oxidative coupling of methane mediated by Sr2Fe1.5Mo0.5O6−δ and its chemical stability. Commun. Chem. 4, 1–9 (2021).
Lu, Z. G. & Zhu, J. H. Thermal evaporation of pure Ag in SOFC-relevant environments. Electrochem. Solid State Lett. 10, B179 (2007).
Bansal, N. P., Salem, J. & Zhu, D. (eds) Advances in Solid Oxide Fuel Cells III: Ceramic and Engineering Science Proceedings Vol. 28 (Wiley, 2007); https://doi.org/10.1002/9780470339534
Nagy, A. J., Mestl, G. & Schlögl, R. The role of subsurface oxygen in the silver-catalyzed, oxidative coupling of methane. J. Catal. 188, 58–68 (1999).
Burnat, D., Nurk, G., Holzer, L., Kopecki, M. & Heel, A. Lanthanum doped strontium titanate–ceria anodes: deconvolution of impedance spectra and relationship with composition and microstructure. J. Power Sources 385, 62–75 (2018).
Gross, M. D., Vohs, J. M. & Gorte, R. J. A strategy for achieving high performance with SOFC ceramic anodes. Electrochem. Solid State Lett. 10, B65 (2007).
Dogu, D. et al. Effect of lanthanum and chlorine doping on strontium titanates for the electrocatalytically-assisted oxidative dehydrogenation of ethane. Appl. Catal. B 227, 90–101 (2018).
Sarsani, S., West, D., Liang, W. & Balakotaiah, V. Autothermal oxidative coupling of methane with ambient feed temperature. Chem. Eng. J. 328, 484–496 (2017).
Sarkar, B., West, D. H. & Balakotaiah, V. Bifurcation analysis of oxidative coupling of methane in monolith, gauze or wire-mesh reactors. Catal. Today https://doi.org/10.1016/j.cattod.2020.12.040 (2021).
Dautzenberg, F. M., Schlatter, J. C., Fox, J. M., Rostrup-Nielsen, J. R. & Christiansen, L. J. Catalyst and reactor requirements for the oxidative coupling of methane. Catal. Today 13, 503–509 (1992).
Vandewalle, L. A., Marin, G. B. & Van Geem, K. M. CFD-based assessment of steady-state multiplicity in a gas–solid vortex reactor for oxidative coupling of methane. Chem. Eng. Process. Process Intensification 165, 108434 (2021).
Tharakaraman, S. S. et al. Development of an active and mechanically stable catalyst for the oxidative coupling of methane in a gas–solid vortex reactor. Ind. Eng. Chem. Res. 61, 7748–7759 (2022).
Zeng, Z. et al. A review of heat transfer and thermal management methods for temperature gradient reduction in solid oxide fuel cell (SOFC) stacks. Appl. Energy 280, 115899 (2020).
Cao, Z. et al. Simultaneous overcome of the equilibrium limitations in BSCF oxygen-permeable membrane reactors: water splitting and methane coupling. Catal. Today 193, 2–7 (2012).
Iftikhar, S. et al. LaNixFe1−xO3 as flexible oxygen or carbon carriers for tunable syngas production and CO2 utilization. Catal. Today 416, 113854 (2023).
Fleischer, V. et al. Investigation of the role of the Na2WO4/Mn/SiO2 catalyst composition in the oxidative coupling of methane by chemical looping experiments. J. Catal. 360, 102–117 (2018).
Zhao, K. et al. Lithium carbonate-promoted mixed rare earth oxides as a generalized strategy for oxidative coupling of methane with exceptional yields. Nat. Commun. 14, 7749 (2023).
Neal, L. M., Yusuf, S., Sofranko, J. A. & Li, F. Oxidative dehydrogenation of ethane: a chemical looping approach. Energy Technology 4, 1200–1208 (2016).
Motagamwala, A. H., Almallahi, R., Wortman, J., Igenegbai, V. O. & Linic, S. Stable and selective catalysts for propane dehydrogenation operating at thermodynamic limit. Science 373, 217–222 (2021).
Saerens, S. et al. The positive role of hydrogen on the dehydrogenation of propane on Pt (111). ACS Catal. 7, 7495–7508 (2017).
Sattler, J. J., Ruiz-Martinez, J., Santillan-Jimenez, E. & Weckhuysen, B. M. Catalytic dehydrogenation of light alkanes on metals and metal oxides. Chem. Rev. 114, 10613–10653 (2014).
Sattler, A. et al. Catalytic limitations on alkane dehydrogenation under H2 deficient conditions relevant to membrane reactors. Energy Environ. Sci. 15, 2120–2129 (2022).
Ziaka, Z., Minet, R. & Tsotsis, T. Propane dehydrogenation in a packed-bed membrane reactor. AIChE J. 39, 3 (1993).
Gbenedio, E., Wu, Z., Hatim, I., Kingsbury, B. F. & Li, K. A multifunctional Pd/alumina hollow fibre membrane reactor for propane dehydrogenation. Catal. Today 156, 93–99 (2010).
Schäfer, R., Noack, M., Kölsch, P., Stöhr, M. & Caro, J. Comparison of different catalysts in the membrane-supported dehydrogenation of propane. Catal. Today 82, 15–23 (2003).
Weyten, H., Keizer, K., Kinoo, A., Luyten, J. & Leysen, R. Dehydrogenation of propane using a packed‐bed catalytic membrane reactor. AlChE J. 43, 1819–1827 (1997).
Borry, R. W. III, Lu, C., Kim, Y.-H. & Iglesia, E. Non-oxidative catalytic conversion of methane with continuous hydrogen removal. Stud. Surf. Sci. Catal. 119, 403–410 (1998).
Sakbodin, M., Wu, Y., Oh, S. C., Wachsman, E. D. & Liu, D. Hydrogen‐permeable tubular membrane reactor: promoting conversion and product selectivity for non‐oxidative activation of methane over an Fe©SiO2 catalyst. Angew. Chem. 128, 16383–16386 (2016).
Liu, L., Liu, D. & Zhang, C. High-temperature hydrogen/propane separations in asymmetric carbon molecular sieve hollow fiber membranes. J. Membr. Sci. 642, 119978 (2022).
Wang, Z. et al. High H2 permeable SAPO-34 hollow fiber membrane for high temperature propane dehydrogenation application. AIChE J. 66, e16278 (2020).
De Vos, R. M. & Verweij, H. High-selectivity, high-flux silica membranes for gas separation. Science 279, 1710–1711 (1998).
Kanezashi, M. & Asaeda, M. Hydrogen permeation characteristics and stability of Ni-doped silica membranes in steam at high temperature. J. Membr. Sci. 271, 86–93 (2006).
Choi, S. et al. Modeling and process simulation of hollow fiber membrane reactor systems for propane dehydrogenation. AlChE J. 63, 4519–4531 (2017).
van Veen, H. M., Bracht, M., Hamoen, E. & Alderliesten, P. T. in Membrane Science and Technology Vol. 4 (eds Burggraaf, A. J. & Cot, L.) 641–680 (Elsevier, 1996).
Choi, S. et al. Material properties and operating configurations of membrane reactors for propane dehydrogenation. AlChE J. 61, 922–935 (2015).
Wismann, S. T. et al. Electrified methane reforming: a compact approach to greener industrial hydrogen production. Science 364, 756–759 (2019).
Weng, G. et al. A high-efficiency electrochemical proton-conducting membrane reactor for ammonia production at intermediate temperatures. Joule 7, 1333–1346 (2023).
Liu, L. et al. Alkane dehydrogenation in scalable and electrifiable carbon membrane reactor. Cell. Rep. Phys Sci. 4, 12 (2023).
Liu, S., Tan, X., Shao, Z. & Diniz da Costa, J. C. Ba0.5Sr0.5Co0.8Fe0.2O3−δ ceramic hollow-fiber membranes for oxygen permeation. AlChE J. 52, 3452–3461 (2006).
Chi, Y., Li, T., Wang, B., Wu, Z. & Li, K. Morphology, performance and stability of multi-bore capillary La0.6Sr0.4Co0.2Fe0.8O3−δ oxygen transport membranes. J. Membr. Sci. 529, 224–233 (2017).
Acknowledgements
This work was supported by the US Department of Energy, Office of Basic Energy Sciences, Division of Chemical Sciences (DE-SC0021008).
Author information
Authors and Affiliations
Contributions
J.W. and S.L. conceived of the concept of this Perspective. J. Zhao and J. Zhang produced figures. J.W. and S.L. wrote the paper. All authors edited and revised this paper. S.L. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Engineering thanks Theo T. Tsotsis, Weishen Yang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wortman, J., Zhao, J., Zhang, J. et al. Multifunctional membrane–catalyst systems for chemical upgrading of shale gas. Nat Chem Eng 2, 539–550 (2025). https://doi.org/10.1038/s44286-025-00252-4
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s44286-025-00252-4