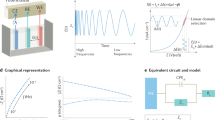
Abstract
Electrochemical impedance spectroscopy (EIS) is a well‐established electrochemical technique that provides invaluable information regarding the properties and functionality of electrodes within bioelectronic devices. EIS is the primary technique that reports on electrode properties in vivo using the implanted device itself. Nevertheless, there are many inconsistencies in the way this technique is implemented and reported on. Without a clear understanding of the experiment and experimental set‐up, it is challenging to draw meaningful conclusions and for results to be extrapolated across studies to benefit and advance the field. This Review discusses in vivo EIS experiments, specifically focusing on challenges in the experimental set‐up, the equipment used, data presentation and circuit modelling for neural interfaces. We propose guidelines for methodical reporting and a consistent, standardized use of terminology, paramount in understanding the performance of electrodes functioning at neural interfaces and promoting the transferability of findings across studies.
Key points
-
Electrochemical impedance spectroscopy is a valuable tool for evaluating the stability of electrodes at a neural interface.
-
Consideration of the equipment limitations and electrochemical set-up (electrode configuration and locations) is critical for meaningful interpretation of impedance measurements.
-
Caution is advised when drawing conclusions from a single frequency measurement. It is recommended that the linearity assumption is supported by an accompanying full-spectrum measurement or a Lissajous plot.
-
Models used in the electrical circuit modelling of impedance spectra should be validated and all elements should represent physical properties at the electrode interface.
-
The development of an on-the-probe reference electrode would enable more robust in vivo measurements and the ability to separate electrode surface changes from tissue impedance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hatsopoulos, N. G. & Donoghue, J. P. The science of neural interface systems. Annu. Rev. Neurosci. 32, 249–266 (2009).
Macdonald, D. D. Reflections on the history of electrochemical impedance spectroscopy. Electrochim. Acta 51, 1376–1388 (2006).
Orazem, M. E. & Tribollet, B. A tutorial on electrochemical impedance spectroscopy. ChemTexts 6, 12 (2020).
Macdonald, J. R. Impedance spectroscopy. Ann. Biomed. Eng. 20, 289–305 (1992).
Kemp, N. T. A tutorial on electrochemical impedance spectroscopy and nanogap electrodes for biosensing applications. IEEE Sens. J. 21, 22232–22245 (2021).
Gabrielli, C. Once upon a time there was EIS. Electrochim. Acta 331, 135324 (2020).
Amini, M., Hisdal, J. & Kalvoy, H. Applications of bioimpedance measurement techniques in tissue engineering. J. Electr. Bioimpedance 9, 142–158 (2018).
Macdonald, J. R. & Johnson, W. B. in Impedance Spectroscopy 1–20 (Wiley, 2018).
Wang, S. et al. Electrochemical impedance spectroscopy. Nat. Rev. Methods Primers 1, 41 (2021). This review discusses the impedance spectroscopy experiment, providing a strong foundation for any application.
Lazanas, A. C. & Prodromidis, M. I. Electrochemical impedance spectroscopy—a tutorial. ACS Meas. Sci. Au 3, 162–193 (2023).
Boukamp, B. A. A linear Kronig–Kramers transform test for immittance data validation. J. Electrochem. Soc. 142, 1885 (1995).
Orazem, M. E., Pébère, N. & Tribollet, B. Enhanced graphical representation of electrochemical impedance data. J. Electrochem. Soc. 153, B129 (2006).
Magar, H. S., Hassan, R. Y. A. & Mulchandani, A. Electrochemical impedance spectroscopy (EIS): principles, construction, and biosensing applications. Sensors 21, 6578 (2021).
Cogan, S. F. Neural stimulation and recording electrodes. Annu. Rev. Biomed. Eng. 10, 275–309 (2008).
Cogan, S. F., Troyk, P. R., Ehrlich, J., Gasbarro, C. M. & Plante, T. D. The influence of electrolyte composition on the in vitro charge-injection limits of activated iridium oxide (AIROF) stimulation electrodes. J. Neural Eng. 4, 79 (2007).
Bobacka, J., Lewenstam, A. & Ivaska, A. Electrochemical impedance spectroscopy of oxidized poly(3,4-ethylenedioxythiophene) film electrodes in aqueous solutions. J. Electroanalytical Chem. 489, 17–27 (2000).
Lempka, S. F., Miocinovic, S., Johnson, M. D., Vitek, J. L. & McIntyre, C. C. In vivo impedance spectroscopy of deep brain stimulation electrodes. J. Neural Eng. 6, 046001 (2009).
Weiland, J. D. & Anderson, D. J. Chronic neural stimulation with thin-film, iridium oxide electrodes. IEEE Trans. Biomed. Eng. 47, 911–918 (2000).
Mercanzini, A., Colin, P., Bensadoun, J. C., Bertsch, A. & Renaud, P. In vivo electrical impedance spectroscopy of tissue reaction to microelectrode arrays. IEEE Trans. Biomed. Eng. 56, 1909–1918 (2009).
Shinwari, M. W. et al. Microfabricated reference electrodes and their biosensing applications. Sensors 10, 1679–1715 (2010).
Krukiewicz, K. Electrochemical impedance spectroscopy as a versatile tool for the characterization of neural tissue: a mini review. Electrochem. Commun. 116, 106742 (2020).
Williams, J. C., Hippensteel, J. A., Dilgen, J., Shain, W. & Kipke, D. R. Complex impedance spectroscopy for monitoring tissue responses to inserted neural implants. J. Neural Eng. 4, 410 (2007). This study analyses full spectrum impedance recorded in vivo and provides evidence of tissue impedance represented by a semicircular arc at high frequencies.
Otte, E., Vlachos, A. & Asplund, M. Engineering strategies towards overcoming bleeding and glial scar formation around neural probes. Cell Tissue Res. 387, 461–477 (2022). This review discusses different neural probes and the biological challenges of implantation and maintenance of the devices on chronic timescales.
Joshi-Imre, A. et al. Chronic recording and electrochemical performance of amorphous silicon carbide-coated Utah electrode arrays implanted in rat motor cortex. J. Neural Eng. 16, 046006 (2019).
Straka, M. M., Shafer, B., Vasudevan, S., Welle, C. & Rieth, L. Characterizing longitudinal changes in the impedance spectra of in-vivo peripheral nerve electrodes. Micromachines 9, 587 (2018).
Cody, P. A., Eles, J. R., Lagenaur, C. F., Kozai, T. D. Y. & Cui, X. T. Unique electrophysiological and impedance signatures between encapsulation types: an analysis of biological Utah array failure and benefit of a biomimetic coating in a rat model. Biomaterials 161, 117–128 (2018). This manuscript evaluates full spectrum impedance measurements alongside histology, discussing the effect of different lead wire encapsulation types.
Ward, M. P., Rajdev, P., Ellison, C. & Irazoqui, P. P. Toward a comparison of microelectrodes for acute and chronic recordings. Brain Res. 1282, 183–200 (2009).
Malaga, K. A. et al. Data-driven model comparing the effects of glial scarring and interface interactions on chronic neural recordings in non-human primates. J. Neural Eng. 13, 016010 (2016).
Barrese, J. C. et al. Failure mode analysis of silicon-based intracortical microelectrode arrays in non-human primates. J. Neural Eng. 10, 066014 (2013).
Ludwig, K. A., Uram, J. D., Yang, J., Martin, D. C. & Kipke, D. R. Chronic neural recordings using silicon microelectrode arrays electrochemically deposited with a poly(3,4-ethylenedioxythiophene) (PEDOT) film. J. Neural Eng. 3, 59–70 (2006).
Vetter, R. J., Williams, J. C., Hetke, J. F., Nunamaker, E. A. & Kipke, D. R. Chronic neural recording using silicon-substrate microelectrode arrays implanted in cerebral cortex. IEEE Trans. Biomed. Eng. 51, 896–904 (2004).
Bodart, C. et al. Electropolymerized poly(3,4-ethylenedioxythiophene) (PEDOT) coatings for implantable deep-brain-stimulating microelectrodes. ACS Appl. Mater. Interfaces 11, 17226–17233 (2019).
Boehler, C. et al. Actively controlled release of dexamethasone from neural microelectrodes in a chronic in vivo study. Biomaterials 129, 176–187 (2017).
Vomero, M. et al. On the longevity of flexible neural interfaces: establishing biostability of polyimide-based intracortical implants. Biomaterials 281, 121372 (2022).
Hughes, C. L. et al. Neural stimulation and recording performance in human sensorimotor cortex over 1500 days. J. Neural Eng. 18, 045012 (2021).
Wang, L. et al. Self-spreadable octopus-like electrode arrays for long-term neural recordings. Acta Phys. Sin. 36, 1909035 (2020).
Cassar, I. R. et al. Electrodeposited platinum–iridium coating improves in vivo recording performance of chronically implanted microelectrode arrays. Biomaterials 205, 120–132 (2019).
Sankar, V. et al. Electrode impedance analysis of chronic tungsten microwire neural implants: understanding abiotic vs. biotic contributions. Front. Neuroeng. 7, 13 (2014).
Prasad, A. et al. Abiotic–biotic characterization of Pt/Ir microelectrode arrays in chronic implants. Front. Neuroeng. 7, 2 (2014).
Wang, C. et al. Characteristics of electrode impedance and stimulation efficacy of a chronic cortical implant using novel annulus electrodes in rat motor cortex. J. Neural Eng. 10, 046010 (2013).
McConnell, G. C., Butera, R. J. & Bellamkonda, R. V. Bioimpedance modeling to monitor astrocytic response to chronically implanted electrodes. J. Neural Eng. 6, 055005 (2009).
Prasad, A. & Sanchez, J. C. Quantifying long-term microelectrode array functionality using chronic in vivo impedance testing. J. Neural Eng. 9, 026028 (2012).
Torres-Martinez, N. et al. Reliability of parylene-based multi-electrode arrays chronically implanted in adult rat brains, and evidence of electrical stimulation on contact impedance. J. Neural Eng. 16, 066047 (2019).
Purcell, E. K., Thompson, D. E., Ludwig, K. A. & Kipke, D. R. Flavopiridol reduces the impedance of neural prostheses in vivo without affecting recording quality. J. Neurosci. Methods 183, 149–157 (2009).
Kane, S. R. et al. Electrical performance of penetrating microelectrodes chronically implanted in cat cortex. IEEE Trans. Biomed. Eng. 60, 2153–2160 (2013).
Cvancara, P. et al. Stability of flexible thin-film metallization stimulation electrodes: analysis of explants after first-in-human study and improvement of in vivo performance. J. Neural Eng. 17, 046006 (2020).
Seaton, B. T. & Heien, M. L. Biocompatible reference electrodes to enhance chronic electrochemical signal fidelity in vivo. Anal. Bioanal. Chem. 413, 6689–6701 (2021). This review highlights the challenges of chronic in vivo electrochemical experiments and provides options and perspectives on stable REs for improved signals.
Moussy, F. & Harrison, D. J. Prevention of the rapid degradation of subcutaneously implanted Ag/AgCl reference electrodes using polymer coatings. Anal. Chem. 66, 674–679 (1994).
Hashemi, P. et al. Chronically implanted, nafion-coated Ag/AgCl reference electrodes for neurochemical applications. ACS Chem. Neurosci. 2, 658–666 (2011).
Jackson, W. F. & Duling, B. R. Toxic effects of silver–silver chloride electrodes on vascular smooth muscle. Circulation Res. 53, 105–108 (1983).
Robbins, E. M., Castagnola, E. & Cui, X. T. Accurate and stable chronic in vivo voltammetry enabled by a replaceable subcutaneous reference electrode. iScience 25, 104845 (2022).
Zhang, F. et al. Reference and counter electrode positions affect electrochemical characterization of bioanodes in different bioelectrochemical systems. Biotechnol. Bioeng. 111, 1931–1939 (2014).
Yang, H. et al. An iridium oxide reference electrode for use in microfabricated biosensors and biochips. Lab Chip 4, 42–46 (2004).
Fan, B. et al. Flexible, diamond-based microelectrodes fabricated using the diamond growth side for neural sensing. Microsyst. Nanoeng. 6, 42 (2020).
Bard, A. J., Faulkner, L. R. & White, H. S. Electrochemical Methods: Fundamentals and Applications 3rd edn (Wiley, 2022).
Orazem, M. E. & Tribollet, B. in Electrochemical Impedance Spectroscopy (eds Orazem, M. E. & Tribollet, B.) 165–189 (Wiley, 2017).
Orazem, M. E. & Tribollet, B. in Electrochemical Impedance Spectroscopy (eds Orazem, M. E. & Tribollet, B.) 139–164 (Wiley, 2017).
Mierzejewski, M., Steins, H., Kshirsagar, P. & Jones, P. D. The noise and impedance of microelectrodes. J. Neural Eng. 17, 052001 (2020).
Boehler, C., Carli, S., Fadiga, L., Stieglitz, T. & Asplund, M. Tutorial: guidelines for standardized performance tests for electrodes intended for neural interfaces and bioelectronics. Nat. Protoc. 15, 3557–3578 (2020). This tutorial-style review provides guidelines for a range of standardized electrochemical tests, including EIS, for electrodes to be used at neural interfaces.
Goh, J., Eluagu, C., Babauta, J. & Orazem, M. Comparison of approaches for assessing linearity of impedance measurements. J. Electrochem. Soc. 171, 036508 (2024).
Liu, X. et al. Stable, long-term single-neuronal recording from the rat spinal cord with flexible carbon nanotube fiber electrodes. J. Neural Eng. 19, 056024 (2022).
Jiang, J., Willett, F. R. & Taylor, D. M. Relationship between microelectrode array impedance and chronic recording quality of single units and local field potentials. In 36th Annual International Conf. IEEE Engineering in Medicine and Biology Society 3045–3048 (IEEE, 2014).
Nolta, N. F., Christensen, M. B., Crane, P. D., Skousen, J. L. & Tresco, P. A. BBB leakage, astrogliosis, and tissue loss correlate with silicon microelectrode array recording performance. Biomaterials 53, 753–762 (2015).
Wellman, S. M. et al. A materials roadmap to functional neural interface design. Adv. Funct. Mater. 28, 1701269 (2018).
Jorfi, M., Skousen, J. L., Weder, C. & Capadona, J. R. Progress towards biocompatible intracortical microelectrodes for neural interfacing applications. J. Neural Eng. 12, 011001 (2015).
Takmakov, P. et al. Rapid evaluation of the durability of cortical neural implants using accelerated aging with reactive oxygen species. J. Neural Eng. 12, 026003 (2015).
Lewis, C. M. et al. Recording quality is systematically related to electrode impedance. Adv. Healthc. Mat. 13, 2303401 (2024).
Ulgut, B. Methods-employing multisine electrochemical impedance spectroscopy for batteries in galvanostatic mode. J. Electrochem. Soc. 169, 110510 (2022).
Fortin, P., Gerhardt, M. R., Ulleberg, Ø., Zenith, F. & Holm, T. Multi-sine EIS for early detection of PEMFC failure modes. Front. Energy Res. 10, 855985 (2022).
You, C., Zabara, M. A., Orazem, M. E. & Ulgut, B. Application of the Kramers–Kronig relations to multi-sine electrochemical impedance measurements. J. Electrochem. Soc. 167, 020515 (2020).
Kozai, T. D., Vazquez, A. L., Weaver, C. L., Kim, S. G. & Cui, X. T. In vivo two-photon microscopy reveals immediate microglial reaction to implantation of microelectrode through extension of processes. J. Neural Eng. 9, 066001 (2012).
Tresco, P. A. & Winslow, B. D. The challenge of integrating devices into the central nervous system. Crit. Rev. Biomed. Eng. 39, 29–44 (2011).
Polikov, V. S., Tresco, P. A. & Reichert, W. M. Response of brain tissue to chronically implanted neural electrodes. J. Neurosci. Methods 148, 1–18 (2005).
Carnicer-Lombarte, A., Chen, S.-T., Malliaras, G. G. & Barone, D. G. Foreign body reaction to implanted biomaterials and its impact in nerve neuroprosthetics. Front. Bioeng. Biotechnol. 9, 622524 (2021).
Bixler, G. & Bhushan, B. Review article: Biofouling: lessons from nature. Philos. Trans. A Math. Phys. Eng. Sci. 370, 2381–2417 (2012).
Biran, R., Martin, D. C. & Tresco, P. A. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp. Neurol. 195, 115–126 (2005).
McConnell, G. C. et al. Implanted neural electrodes cause chronic, local inflammation that is correlated with local neurodegeneration. J. Neural Eng. 6, 056003 (2009).
Usoro, J. O., Sturgill, B. S., Musselman, K. C., Capadona, J. R. & Pancrazio, J. J. Intracortical microelectrode array unit yield under chronic conditions: a comparative evaluation. Micromachines 12, 972 (2021).
Meijs, S. et al. Influence of fibrous encapsulation on electro-chemical properties of TiN electrodes. Med. Eng. Phys. 38, 468–476 (2016).
Woeppel, K. et al. Explant analysis of Utah electrode arrays implanted in human cortex for brain–computer interfaces. Fronti. Bioeng. Biotechnolol. 9, 759711 (2021).
Evers, J. & Lowery, M. The active electrode in the living brain: the response of the brain parenchyma to chronically implanted deep brain stimulation electrodes. Operative Neurosurg. 20, 131–140 (2021).
Kilias, A. et al. Intracortical probe arrays with silicon backbone and microelectrodes on thin polyimide wings enable long-term stable recordings in vivo. J. Neural Eng. 18, 066026 (2021).
Steins, H. et al. A flexible protruding microelectrode array for neural interfacing in bioelectronic medicine. Microsyst. Nanoeng. 8, 131 (2022).
Zhou, T. et al. Syringe-injectable mesh electronics integrate seamlessly with minimal chronic immune response in the brain. Proc. Natl Acad. Sci. USA 114, 5894–5899 (2017).
Ferguson, M., Sharma, D., Ross, D. & Zhao, F. A critical review of microelectrode arrays and strategies for improving neural interfaces. Adv. Healthc. Mater. 8, e1900558 (2019).
O’Sullivan, K. P., Orazem, M. E., Otto, K. J., Butson, C. R. & Baker, J. L. Electrical rejuvenation of chronically implanted macroelectrodes in nonhuman primates. J. Neural Eng. 21, 036056 (2024).
Otto, K. J., Johnson, M. D. & Kipke, D. R. Voltage pulses change neural interface properties and improve unit recordings with chronically implanted microelectrodes. IEEE Trans. Biomed. Eng. 53, 333–340 (2006).
Johnson, M. D., Otto, K. J. & Kipke, D. R. Repeated voltage biasing improves unit recordings by reducing resistive tissue impedances. IEEE Trans. Neural Syst. Rehabil. Eng. 13, 160–165 (2005).
Evers, J. et al. Stimulation-induced changes at the electrode–tissue interface and their influence on deep brain stimulation. J. Neural Eng. 19, 046004 (2022).
Hemm, S. et al. Evolution of brain impedance in dystonic patients treated by GPi electrical stimulation. Neuromodulation 7, 67–75 (2004).
Minteer, S. et al. New guidelines for presenting electrochemical data in all ACS journals. J. Org. Chem. 88, 4036–4037 (2023).
Vomero, M. et al. Conformable polyimide-based μECoGs: bringing the electrodes closer to the signal source. Biomaterials 255, 120178 (2020).
Acknowledgements
This work was supported by the CatWalk Spinal Cord Injury Trust and the Health Research Council of New Zealand (project grant and HRC/Catwalk Partnership 19/895). This work was also supported by the Assistant Secretary of Defense for Health Affairs endorsed by the Department of Defense (US $534,258) through the Spinal Cord Injury Research Program (award HT9425-23-1-0492). Opinions, interpretations, conclusions and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense. B. Hazelgrove was supported by a University of Auckland Doctoral Scholarship. B.R. was supported by the Neurological Foundation First Fellowship (1952 FF). M.A. and L.M were supported by the European Research Council (ERC) under the European Union’s Horizon 2020 Research and Innovation programme (grant agreement 101113487 ‘ProBER‘), the European Innovation Council H2020 programme (grant agreements 899287 ‘NeuraViper’ and 101099366 ‘BioFINE’), and the Chalmers Gender Initiative for Excellence (GENIE).
Author information
Authors and Affiliations
Contributions
B. Hazelgrove and L.M. researched data for the article. All authors substantially contributed to the discussion of the content, wrote the manuscript, and reviewed and edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Electrical Engineering thanks Alexandra Joshi-Imre, who co-reviewed with Yupeng Wu, Katarzyna Krukiewicz and Mark E. Orazem for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Blackrock Neurotech: https://blackrockneurotech.com/
Intan Technologies: https://intantech.com/
Metrohm: https://www.metrohm.com/en_au/products/electrochemistry.html
nanoZ Impedance Tester: https://plexon.com/products/nanoz-impedance-tester/
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hazelgrove, B., Matter, L., Raos, B. et al. Electrochemical impedance spectroscopy in vivo for neurotechnology and bioelectronics. Nat Rev Electr Eng 2, 110–124 (2025). https://doi.org/10.1038/s44287-024-00126-6
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44287-024-00126-6
This article is cited by
-
Daily electric field treatment improves functional outcomes after thoracic contusion spinal cord injury in rats
Nature Communications (2025)