Abstract
Zoonotic viruses rank among the greatest threats to public health, with urbanization and global warming accelerating their emergence and spread. As the risk of future pandemics grows, innovative tools are needed to deepen our understanding of viral pathogenesis and enhance pandemic preparedness. Nonviral protein cages provide a versatile platform for studying viral mechanisms, virus-host interactions, and designing next-generation therapeutic approaches, making them powerful assets in the fight against viral threats.
Similar content being viewed by others
Introduction
In the complex realm of microorganisms, viruses stand out as enigmatic entities, existing at the intersection of life and non-life. Viruses consist of a minimal genome containing the necessary information for replication, which is encapsulated in a protein shell called a capsid, and in some cases, also a lipid envelope derived from the host cell membrane, studded with viral glycoproteins that mediate host recognition and entry.
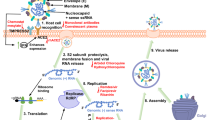
Viral infection begins with a multistep entry process, where the virus first attaches to host cell surface receptors and penetrates the cell through membrane fusion or endocytosis (Fig. 1). Virions that enter endosomes escape in response to environmental signals such as pH changes or enzyme activation. Once inside the cytosol, the viral capsid undergoes uncoating, releasing its genetic material for replication. Subsequent stages in the life cycle include the synthesis of viral components, followed by virion assembly and release. As obligate intracellular parasites, viruses rely on the host cell’s machinery for propagation. Understanding which host factors are recruited throughout the viral life cycle is crucial, as these interactions drive viral pathogenicity and can inform therapeutic strategies to combat emerging threats. However, studying these mechanisms using live viruses presents significant challenges. Viral entry often involves multiple receptors and attachment factors, making it difficult to isolate specific interactions. Moreover, modifying viral surface properties to study these processes can be inherently limited by strict biosafety regulations, which further restrict research accessibility when handling highly pathogenic viruses.
The majority of viruses—whether enveloped or nonenveloped—depend on the host cell’s endocytic pathways for entry (left)77,163,164,165,166. Some viruses bypass complex entry mechanisms by directly fusing their envelope with the plasma membrane, allowing their capsids to reach the cytosol (middle). Endocytosed viruses undergo a multistep process that moves them from the cell surface to specific intracellular sites where their genetic material is released. Initially, viruses attach to cellular receptors, which may be proteins, lipids, or carbohydrates, typically in a specific and multivalent fashion. These interactions trigger cellular signaling pathways, leading to virus uptake through various endocytic mechanisms, primarily macropinocytosis and clathrin-mediated endocytosis. Following vesicular trafficking, host cell cues—such as pH changes or proteolytic cleavage—induce conformational changes in the virus, facilitating penetration into the cytosol. Enveloped viruses achieve this through membrane fusion, while nonenveloped viruses disrupt the vacuolar membrane through pore formation or lysis. The precise site of penetration varies depending on the virus and host cell type, occurring at the plasma membrane, early or late endosomes, macropinosomes, or the endoplasmic reticulum. After cytosolic entry, RNA viruses typically replicate in the cytoplasm, whereas most DNA viruses continue their journey to the nucleus. The entry process concludes with controlled uncoating of the viral genome, marking the start of replication (right). Newly synthesized viral components are then assembled into progeny virions, which are subsequently released from the host cell via budding or lysis.
To address these challenges, cages derived from nonviral proteins are a promising class of tools for studying viruses. These hollow particles are well-defined structures that can mimic the architecture of non-enveloped viruses, eliminating the need to work with live, infectious agents. Their versatility allows them to be engineered to imitate specific viral functions in controlled settings1,2, providing a platform to dissect virus-host interactions and cellular responses without the complexity associated with studying whole viruses. Additionally, the low inherent toxicity of proteins and their inability to replicate make them safer and more accessible for exploratory research in laboratory settings.
In this perspective, engineering efforts aimed at mimicking various stages of the viral life cycle using artificial protein cages are briefly reviewed. While numerous examples of virus-like particles have been described in the literature, this article focuses exclusively on scaffolds derived from nonviral proteins, such as ferritin, lumazine synthase, and computationally designed architectures (Fig. 2). Through engineering to exhibit virus-like behaviors, these artificial systems offer valuable insights into viral processes1 and present new opportunities to develop innovative medical and biotechnological solutions to address current and future health challenges2.
Surface representations of different protein cages varying in size, symmetry, and the number of constituent capsomers, derived from either naturally occurring (a–d) or computationally designed scaffolds (e–i). a AaLS-wt (PDB 5MPP), a 60-subunit self-assembling cage, served as a template for multiple directed evolution campaigns. b NC-4 (PDB 7A4J) and c AaLS-13 (PDB 5MQ7) are evolved variants of AaLS-wt, optimized to encapsulate their own encoding mRNA55 and toxic proteins167, respectively, with both exhibiting increased diameters. d A variant of human heavy-chain ferritin (PDB 2CEI) with positively charged surface residues was encapsulated within AaLS-13, forming matryoshka-like assemblies168. e OP (PDB 6FDB) was engineered by introducing positive charges onto the lumenal surface of the computationally designed O3-33 cage43. Owing to its positively charged lumen and porous architecture, OP can be efficiently loaded in vitro with a desired oligonucleotide44 or anionic surfactants, forming a hydrophobic core that enables the sequestration of non-polar molecules45. f I3-01 is a 60-subunit icosahedral cage composed exclusively of trimeric subunits. Its variant, mi3, was created by mutating surface-exposed cysteines (Cys76 and Cys100) to alanine169. The SpyTag/SpyCatcher technology170 has been used to encapsulate both proteinaceous and non-proteinaceous cargo within mi3 cages13. g I53-50 (PDB 7SGE) is a 120-subunit icosahedral assembly comprising trimeric (blue) and pentameric (gray) components171. I53-50 has been engineered to encapsulate nucleic acids52 as well as drug-loaded synthetic polymers63. h O42.1 is a 30 nm porous octahedral structure consisting of six tetramers (gray) and 12 dimeric Fc subunits (blue). i Closure of the O42.1 cage’s pores with pH-responsive trimeric plugs (yellow) resulted in the O432 variant. Modifying the interior-facing residues of the trimeric plug to introduce internal charge enabled the packaging of nucleic acid or protein cargo172. All structures were visualized using ChimeraX173,174. O42.1 and O432 were modeled from density maps deposited in the Electron Microscopy Data Bank175 (EMD-29602).
Binding, internalization, and cargo release
While human ferritin exhibits low-affinity binding to transferrin receptor-1, which mediates its internalization3, nonviral protein cages (NVPCs) typically lack inherent membrane-binding capabilities. However, surface engineering can enhance their affinity for cellular membranes. For instance, the trans-activator of transcription (Tat) peptide from the human immunodeficiency virus (HIV)4 is widely employed to facilitate the cellular uptake of protein cages. Its ability to penetrate cell membranes arises from its positively charged, arginine- and lysine-rich sequence, which interacts with negatively charged cell-surface molecules such as heparin sulfate. This enables Tat-fused proteins to cross cellular barriers in an endocytosis-independent manner5,6. In contrast, the tripeptide arginine-glycine-aspartic acid (RGD), found in the adenovirus penton base protein7, is critical for receptor-mediated viral entry as it interacts with αv integrins on host cells, triggering endocytosis8. To enhance cell binding and uptake, RGD motifs have been incorporated into protein cages such as lumazine synthase9, ferritin10,11,12, and a a variant of the computationally designed I3-01 cage, known as mi313. While RGD peptides are effective in targeting integrins, full-length antibodies14,15,16,17, antibody fragments18,19, and mimics20 have also been used to direct protein cages to alternative receptors.
Antibody-binding domains (ABDs)21, such as those derived from protein A22,23, are often employed to display antibodies on the surface of NVPCs24,25,26,27,28,29,30,31. These domains specifically bind to the Fc region of antibodies, ensuring proper orientation of the Fab regions for receptor recognition. Using this method, variants of lumazine synthase have been successfully targeted to cells overexpressing CD4432 or the human epidermal growth factor receptor 232,33, with minimal uptake observed in receptor-negative cells. However, the non-covalent nature of ABD-antibody interactions can result in loss of targeting specificity in the presence of competing immunoglobulins, restricting this approach to in vitro applications33. Improved targetability in competitive environments could potentially be achieved through covalent linkage of antibodies to the cage or ensuring full antibody occupancy on the cage surface, thereby reducing the likelihood of antibody exchange. However, such strategies may lead to challenges, such as antibodies crosslinking multiple nanoparticles, potentially causing precipitation.
Recent advances in computational protein design have made it possible to precisely orient antibodies in non-natural geometric assemblies. By grafting residues from protein A onto dimeric, trimeric, tetrameric, or pentameric helical repeat proteins, researchers have constructed dihedral, tetrahedral, octahedral, and icosahedral architectures that display 2, 6, 12, and 30 protein A-binding antibodies per cage, respectively34. These antibody nanocages have been successfully targeted to cells overexpressing the epidermal growth factor receptor. However, their performance in the presence of competing immunoglobulins remains uncertain. Furthermore, after internalization, these engineered protein cages tend to become trapped within endosomes, potentially limiting their functional efficacy33,34.
Once internalized via endocytosis, viruses must escape the endolysosomal pathway to avoid degradation, deliver their cargo, and successfully infect the host. Although no universal escape mechanism exists, a common feature is the exploitation of endosomal acidification. As the endosome matures, its pH gradually decreases due to the activity of ATP-dependent proton pumps35. Endosomal acidification serves as a crucial trigger for conformational changes in viral proteins, facilitating their interaction with the endosomal membrane36. A prominent example is the influenza A virus, where pH-driven changes in hemagglutinin enable membrane fusion and genome release37,38. Additionally, the influenza M2 protein forms a proton channel that allows protons to flow into the virus interior, further promoting uncoating39,40.
In contrast to their enveloped counterparts, non-enveloped viruses cannot fuse with the endosomal membrane. To gain access to the cytoplasm, they disrupt endosomes through lysis or pore formation processes that remain incompletely understood41. Mimicking these mechanisms using membrane-less protein cages presents a significant challenge. Although a general strategy for achieving endosomal escape has yet to be established, the pH-responsive behavior of viruses has inspired engineering efforts. For example, researchers designed trimeric “plug” elements to seal large pores in octahedral antibody nanoparticles42. These plugs remain closed at neutral pH but dissociate in vitro at a pH of 6.7—higher than the typical endosomal pH, which ranges from 6.5 (early endosome) to 5.5 (late endosome).
In studies on an engineered variant of the computationally designed O3-33 cage43, known as OP44,45,46, researchers attributed cytoplasmic siRNA delivery to a hexahistidine tag on each of the cage’s 24 subunits44. Hypothetically, protonation of the histidine residues induces an osmotic imbalance, leading to water influx and endosomal swelling—a phenomenon known as the proton sponge effect47. This swelling can destabilize the endosomal membrane, ultimately releasing the cargo into the cytoplasm. Alternatively, protonated histidine residues may directly induce endosomal membrane permeabilization48. Engineering such pH-responsive features in artificial cages may bring us closer to replicating intracellular cargo release and enhances our understanding of viral entry processes following endosomal priming, including membrane fusion, uncoating, and genome release.
Nucleic acid packaging
After successful replication, the virus progresses to the late stages of its life cycle, including assembly and release from the host cell. These processes ensure the formation of infectious progeny and their dissemination. During assembly, the newly synthesized viral genome is efficiently encapsulated within the viral capsid. Some viruses achieve remarkable packaging efficiency by utilizing relatively simple recognition motifs49. Many capsid proteins are positively charged, allowing them to bind tightly to the negatively charged phosphate backbone of nucleic acids. Inspired by these natural mechanisms, artificial RNA encapsulation systems using NVPCs have been developed1. By incorporating positively charged residues on the lumenal surfaces of these cages, particles capable of encapsulating endogenous nucleic acids in cellular environments were created44,46,50,51. In the case of stable, porous cages, such as OP, unwanted bacterial RNA guests can be removed using a combination of RNase A treatment to degrade contaminant RNA and high-ionic-strength buffers to weaken electrostatic interactions44. The resulting empty cages can then be loaded in vitro with desired synthetic oligonucleotides. To achieve selective genome packaging in vivo, researchers have employed directed evolution strategies.
In 2017, David Baker’s laboratory—led by the 2024 Nobel Prize laureate in Chemistry—used the computationally designed two-component cage I53-50 as a starting point for evolution52. A library of protein cages with variably charged interior surfaces was generated and selected for genome packaging and cargo protection. After several rounds of evolution, a nucleocapsid (I53-50-v4) with a positively charged inner surface was generated. These evolved protein cages were capable of packaging one full-length genome for every 11 particles. Similarly, in 2018, Hilvert and coworkers utilized the wild-type enzyme lumazine synthase from the hyperthermophilic organism Aquifex aeolicus (AaLS), which naturally forms 60-subunit dodecahedral cages, as a scaffold. By applying circular permutation to the AaLS monomers, the N- and C-termini were repositioned from the exterior to the interior surface53. The λN+ peptide, derived from bacteriophage lambda, was genetically introduced to the newly relocated N-terminus54. This peptide binds the boxB RNA hairpin structure through its arginine-rich domain. By flanking the capsid protein mRNA sequence with boxB tags, assemblies capable of binding their own encoding mRNA were generated and subjected to directed evolution. After several rounds of mutagenesis and screening, the best variant, NC-4, emerged as icosahedrally symmetric closed shells that encapsulated an average of two copies of its coding mRNA per particle55. Remarkably, these cages protected their cargo from nuclease degradation for days. This efficient packaging was achieved through the co-evolution of packaging signals within the RNA genome, mirroring a hallmark of viral evolution55. Structurally, NC-4 adopted an architecture resembling that of T = 4 viruses and featured 3D-domain swaps, which have been reported to enhance the stability of certain viral capsids56,57. NC-4 provides compelling evidence supporting the hypothesis that viruses may have emerged from preexisting cellular components58,59.
Despite their ability to encapsulate their own genetic material with high precision, some viruses package foreign genomes. This process, known as viral reassortment, is particularly prevalent among RNA viruses and occurs when two or more viruses of the same genera with segmented genomes, such as the influenza virus, co-infect a cell and exchange genetic material60. Reassortment of gene segments in influenza viruses can result in significant antigenic changes to the hemagglutinin and neuraminidase proteins, potentially creating chimeric viruses with an increased risk of zoonotic spillover61. The 2009 H1N1 pandemic is a notable example of such a reassortment event62. Understanding this process is vital for improving pandemic preparedness.
Artificial nucleocapsids offer modular and controllable systems to study the fundamental mechanisms underlying viral reassortment. Encapsulation of foreign RNA sequences in vitro has been demonstrated using the nucleocapsids I53-50-v4 and NC-463,64. For I53-50-v4, preventing cage assembly in the cytoplasm of E. coli was achieved by separately producing the pentameric and trimeric components. This approach required a single point mutation to enhance the solubility of the pentameric subunits. Single-stranded RNA molecules of varying sizes were encapsulated simply by mixing the cage components with negatively charged RNA cargo63. Similarly, the assembly of NC-4 cages in cells was inhibited by fusing each monomer to a maltose-binding protein64. Selective proteolytic cleavage of this steric block in the presence of up to 2600 nucleotides of foreign RNA triggered capsid assembly.
The nucleocapsids assembled in vitro exhibited the same structural characteristics as those formed in vivo and efficiently protected their cargo from nuclease degradation. Although replicating genome packaging within a physiologically relevant environment can be challenging, the ability to precisely control cargo composition and encapsulation offers significant advantages for studying viral packaging mechanisms. For instance, it could allow assessment of the encapsulation efficiency of genomes with different packaging signals, aligning with studies using virus-derived proteins65,66,67,68,69,70.
In host cells, viruses can hijack existing biomolecular condensates or even induce new ones to support their life cycle71. For example, the replication and assembly of influenza virus are facilitated by its genome—eight single-stranded, negative-sense RNA segments—undergoing liquid-liquid phase separation72,73,74. Since these biomolecular condensates are fragile and difficult to observe and analyze75, novel characterization methods are highly sought after.
In a recent study, human ferritin and mi3, were engineered to interact with liquid-liquid phase-separated proteins76. Encapsulating the condensates with a stabilizing layer of cages hardened them and prevented coalescence, enabling detailed structural visualization using cryoET. By adjusting the condensate-to-cage ratio, researchers could significantly vary the size of the assembled condensates. Once the manipulation of these membrane-less organelles within cells becomes possible, it could open new avenues for exploring phase-separated viral genomes, such as that of influenza.
Virion release
Virus release from host cells can occur through various mechanisms, including lysis, exocytosis, and budding77. Most enveloped viruses, such as HIV, acquire their envelope via the budding process78. The HIV-1 Gag protein plays a pivotal role in this pathway79. The N-terminal domain of Gag recruits phosphatidylinositol (4,5) bisphosphate at the host plasma membrane80, while the C-terminal p6 region facilitates interactions with the host ESCRT machinery to mediate the final membrane fission step required for release81.
This understanding has been leveraged to engineer artificial NVPCs capable of directing their own release from eukaryotic cells. For instance, the first six amino acids of the HIV-1 Gag protein and its p6 region were genetically fused to the N- and C-termini, respectively, of the computationally designed protein cages I3-01 and O3-3382,83. Expression of these constructs in human embryonic kidney (HEK) 293T cells resulted in the production of extracellular vesicles of varying sizes containing multiple copies of the designed nanocages. Cryo-electron tomography revealed that most of the nanocages were membrane-associated.
In contrast to these nonviral systems, HIV-1 capsids are released individually from the plasma membrane84. This variation in the number of proteinaceous capsids per vesicle highlights the significant influence of factors such as particle size and the surface density of Gag and p6 proteins on the budding process. Adapting this strategy to various scaffolds presents an opportunity to study how these factors affect membrane curvature and the recruitment of host machinery. To gain structural insights into virus-host interactions, cryo-electron tomography provides high-resolution 3D visualizations of macromolecular complexes in their native cellular environment85,86. Unlike polymorphic viruses such as influenza, which are challenging to image due to their variability, protein cages offer a consistent size and high contrast, making them a reliable alternative for structural studies in cells while reducing variability and enhancing data quality.
Lipid envelope coating opens new possibilities for creating innovative types of pseudotyped particles based on nonviral capsids. Traditional pseudotyping involves producing viral vectors with envelope proteins from a different virus to either limit or broaden the range of cell types they can infect, referred to as their tropism87. To generate pseudotyped NVPCs, the engineered I3-01 scaffold was co-expressed with a beta-lactamase fusion protein designed for encapsulation within the cages, along with the vesicular stomatitis virus glycoprotein (VSV-G)83. VSV-G, a trimeric spike protein, is commonly used for pseudotyping as it facilitates receptor binding and membrane fusion88. All three proteins, encoded on separate plasmids, were efficiently co-released in extracellular vesicles from HEK 293T producer cells. After harvesting, the uptake of particles by HeLa cells was assessed using a beta-lactamase colorimetric activity assay, confirming successful cargo release into the host cytoplasm, likely due to VSV-G-mediated membrane fusion at either the plasma or endosomal membrane.
VSV-G-pseudotyped particles are ideal for studying membrane fusion mechanisms, and extending this approach to other spike proteins could enable functional investigations without requiring intact pathogens. Traditional pseudotyping methods allowed virus studies in BSL-2 labs instead of higher-containment BSL-3 or BSL-4 facilities89, which are associated with high costs, limited access, and strict biosafety protocols. These constraints slow research, reduce throughput, and increase logistical complexity. Pseudotyped NVPCs offer a safer alternative, potentially enabling research in BSL-1 labs and broadening participation in viral mechanism studies.
Immune modulation
The viral envelope plays an important role in modulating the immune response against enveloped viruses90,91. By masking underlying viral proteins, the envelope reduces the immune system’s ability to recognize and neutralize the virus. This immune evasion strategy can also be adapted to nonviral systems, as demonstrated by the enveloped I3-01 particles, where the acquired membrane shielded the protein cage from antibody recognition83. Surrounding protein-based compartments with host-derived components appears to be a general mechanism for reducing immunogenicity and appearing less foreign to the immune system. For instance, the previously described AaLS variant displaying ABDs was less immunogenic than its parent scaffold lacking these binding peptides33. This reduction in immunogenicity was attributed to the cage’s recruitment of circulating immunoglobulins, forming a protective shield of host proteins that masked the underlying particle.
Glycans on viral glycoproteins further contribute to immune evasion by mimicking host molecules and obscuring key antigenic sites, thereby preventing detection by neutralizing antibodies92,93,94. Artificial protein cages can be similarly shielded through glycoengineering platforms. For example, an AaLS variant and the computationally designed I53-50-v4 were successfully glucosylated in the cytoplasm of E. coli through co-expression with the asparagine N-glucosyltransferase from Actinobacillus pleuropneumoniae95. As demonstrated with the AP205 bacteriophage, the installed N-linked glucose can serve as a primer for sugar chain elongation95. A particularly promising immune-stealthing modification involves linear polysaccharides composed of α-2,8-linked sialic acid, for which enzymatic pathways have already been engineered96. Alternatively, cell-free biosynthesis platforms offer modular approaches for modifying proteins with diverse oligosaccharides97,98. Moreover, the eukaryotic glycosylation machinery can be exploited to replicate specific viral glycosylation patterns, a key factor in the development of protein-based vaccines.
Training the immune system to recognize pathogens remains one of the most effective strategies to combat infectious diseases. While traditional vaccines using live-attenuated or inactivated viruses have been successful, they pose challenges related to safety99,100. Safer alternatives involve using isolated pathogen antigens to elicit robust immune responses. However, antigen fragments alone are often weakly immunogenic, even when combined with adjuvants101. To enhance immunogenicity, antigen fragments can be multivalently displayed on scaffolding proteins. Protein-based particles improve antigen delivery to lymph nodes—key sites for immune activation—and promote efficient uptake by antigen-presenting cells102,103. Additionally, presenting antigens on nanoparticles enhances B-cell activation by effectively cross-linking B-cell receptors, leading to stronger antibody production102,103.
Although naturally occurring protein cages such as ferritin104 and AaLS105 have been widely used in vaccine development, computational design has greatly expanded the range of available antigen display platforms106. These artificial scaffolds exhibit diverse symmetries and sizes, often exceeding those of natural cages, enabling precise control over antigen number, placement, and spacing107. Additionally, some architectures incorporate two distinct protein components, including a trimeric unit ideal for presenting trimeric antigens, which distinguishes them from homomeric scaffolds like ferritin and AaLS. This feature enables the formation of particles displaying multiple antigens simply by mixing independently produced scaffolding proteins and antigenic fragments. These mosaic designs are under development to create vaccines targeting viruses with overlapping seasons108 or to protect against heterotypic viruses109,110 and diverse coronavirus subgenera111. In contrast, assembling similar constructs with homomeric cages requires additional post-production conjugation techniques, such as SpyTag/SpyCatcher technologies112,113,114.
The two-component icosahedral I53-50 cage is widely used as a vaccine carrier due to its high valency, efficient production, and capacity to display diverse antigens, including the respiratory syncytial virus fusion protein115, influenza hemagglutinin109, and the SARS-CoV-2 spike116 and receptor-binding domain117. However, when presenting heavily glycosylated antigens such as the HIV-1 envelope protein, issues with assembly and stability were observed, likely due to excessive crowding on the cage surface118. Interestingly, using an equimolar amount of the trimeric antigen for immunization, displaying it on a tetrahedral cage produced a more stable construct that elicited an immune response comparable to that of I53-50, despite presenting five times fewer trimers (four versus 20)118. This may be due to the greater epitope accessibility offered by tetrahedral symmetry compared to icosahedral symmetry34.
In a separate study aimed at developing a livestock vaccine for East Coast fever, a deadly disease caused by Theileria parva, researchers compared the immune responses elicited by displaying a parasitic antigen on I53-50 and two other icosahedral cages119. Notably, I53-50 induced a stronger immune response than the other scaffolds. However, one of the alternative cages exclusively triggered an IgG1 response with no detectable IgG2, highlighting differences in immune polarization among these designs.
While these studies provide valuable insights into the immunological characteristics of computationally designed cages, further comparative assessments are necessary to fully determine the advantages of different platforms. Systematic testing of diverse building block arrangements120 could clarify how factors like particle size, antigen valency and spacing, as well as surface properties, affect vaccine potency, guiding the design of more effective formulations.
Future vaccine development will benefit from advancements in computational tools capable of predicting viral glycoprotein mutations that may compromise existing vaccines121. Given that nucleocapsids directly link genotype and phenotype122, it should theoretically be possible to randomize the sequence of displayed viral spikes and select for variants that acquire resistance to neutralizing antibodies in vivo, offering a valuable approach for training prediction tools. Early detection of unusual mutations associated with zoonotic spillovers or emerging pathogens through viral surveillance will also play a crucial role in pandemic prevention123. The ability to design artificial protein cages that display viral glycoproteins with precise geometries presents exciting opportunities. Such constructs could incorporate predicted mutations linked to increased public health risks, enhancing pandemic preparedness by enabling ready-to-use vaccines or providing model systems for a deeper understanding of viral pathogenesis.
Model systems for pathogenicity studies
A significant concern in modern virology is the presence of polybasic cleavage sites (PCSs) in the spike proteins of many phylogenetically distant viruses124. These sequences, usually rich in basic amino acids like arginine or lysine, are targeted and cleaved by host cell proteases, a process essential for virus activation. PCSs are particularly notable in SARS-CoV-2 and MERS-CoV, where they enhance replication in the respiratory tract and human transmissibility125,126,127,128,129. Similarly, PCSs in the hemagglutinin proteins of highly pathogenic avian influenza viruses (e.g., H5 and H7 subtypes) are associated with increased virulence and organ tropism, raising significant concerns about zoonotic transmission130,131,132,133,134,135.
Cleavage of PCSs by furin generates a C-terminal R/KXXR/K motif, known as the C-end rule (CendR) motif, where R and K represent arginine and lysine, and X denotes any amino acid. This motif binds neuropilin (NRP) receptors136, which are transmembrane proteins involved in signaling, angiogenesis, and immune modulation137,138,139. NRPs facilitate cellular entry for viruses bearing CendR motifs140,141,142,143, including SARS-CoV-2144,145; however, the broader role of NRP-CendR interactions in viral pathogenicity remains unclear. Critical questions include whether NRPs facilitate the entry of all PCS-containing viruses (e.g., Ebola), if additional host factors contribute to entry of viruses with CendR peptides, how PCS-mediated internalization impacts viral trafficking, and whether PCSs reflect convergent evolution or influence zoonotic transitions.
Inserting or removing PCSs from viruses could help address these questions, but the process is not straightforward. Removing or altering PCSs may impair viral replication, rendering the virus non-viable, while inserting PCSs could result in unintended outcomes, such as gain-of-function, potentially creating new harmful strains. These concerns underscore the ethical and safety challenges of handling pathogenic viruses. Developing alternative approaches is essential for responsible research. Nonviral self-assembling protein cages offer a promising solution for studying PCSs mechanisms while minimizing risks. By designing protein cages that display PCS-containing spikes or CendR motifs, associated viral entry mechanisms could be investigated in a controlled and safe manner.
Exploring viral processes through imaging techniques can also be significantly simplified using protein cages. They can be engineered to incorporate fluorophores or contrast agents at specific sites146,147 or to encapsulate imaging modalities such as fluorescent proteins148. These labeled particles enable tracking via confocal microscopy to study processes such as endocytosis, trafficking, and intracellular sorting. Proteomics profiling of cage-positive cells isolated through laser microdissection can identify specific host factors involved in the endocytosis of virus-mimicking particles149. Combining this with subcellular fractionation allows for spatial identification of proteins associated with key compartments, such as surface receptors, endosomal markers, or nuclear proteins150,151. Functional validation of these factors can be achieved using small-molecule inhibitors152, RNA interference, or CRISPR-based gene knockouts.
Protein cages are useful scaffolds for cryo-electron microscopy studies due to their uniform size and symmetrical structure, which streamline image processing and enhance the precision of 3D reconstruction153,154. This makes them particularly suitable for examining structural changes resulting from the incorporation of PCSs into viral glycoproteins. For instance, cages engineered to display a mutated hemagglutinin protein with an introduced PCS could reveal structural differences that explain the enhanced virulence of H5 or H7 influenza subtypes in poultry. However, NVPCs are not enveloped and are smaller than influenza viruses, which may limit their ability to fully mimic native viruses. Harnessing pseudotyped nonviral cages with an envelope could overcome these limitations by providing a more virus-like platform. Integrating structural data with phenotypic insights from cage entry assays could establish a comprehensive genotype-structure-phenotype relationship, deepening our understanding of how PCSs in spike proteins influence viral virulence.
Conclusion
Historically, many pandemic-causing infectious diseases originated from animals. The emergence of such life-threatening pathogens is facilitated by urbarnization155, which increases animal-human contact, and global warming156,157,158, which promotes the spread of wildlife and virus vectors like mosquitoes to new geographical areas. Understanding how these pathogens evolve is crucial for developing effective treatments and preventive measures. NVPCs, with their exceptional engineerability, provide a remarkable platform for mimicking and studying viral structure and function. Importantly, these systems eliminate the safety concerns associated with live pathogens or pseudotyped viruses, which require at least biosafety level 2 facilities. By enabling rapid, safe investigations of viral glycoproteins and their mutations, NVPCs can facilitate the early identification of variants with pandemic potential, as well as the host factors that promote their entry.
Despite significant progress, critical questions about viral assembly remain unanswered. How are segmented genomes packaged with one copy of each segment per particle? What roles do viral and host condensates play in this process? Which host factors influence genome packaging efficiency and fidelity? NVPCs offer a unique opportunity to dissect these complex mechanisms by enabling controlled experiments that would be challenging with live viruses. Addressing these questions will not only deepen our understanding of viral biology but also improve our ability to intervene at later stages of the viral lifecycle, thus limiting further spread. Democratizing the use of NVPCs as versatile tools for studying viruses will advance both fundamental research and global preparedness for future pandemics.
The Coalition for Epidemic Preparedness Innovations (CEPI) has identified 25 virus families that are most likely to produce the next deadly viral threat. Leveraging NVPCs to gain insights into these virus families, as well as utilizing them as vaccine platforms, could play a pivotal role in mitigating the impact of future pandemics on global public health. Unlike the lipids used in mRNA vaccines, these particles can be produced through entirely bioprocess-based methods, reducing carbon emissions, chemical waste, and environmental impact. This approach also lowers production costs, making treatment and immunization more affordable and accessible. While large-scale production of NVPCs can benefit from the expertise gained with other protein therapeutics, much remains to be learned to ensure proper cage assembly and purity, while also meeting good manufacturing practice requirements. Establishing efficient manufacturing methods could enable rapid initial production and scalable outputs, key elements in achieving CEPI’s ambitious “100 Days Mission” to respond to and contain future pandemics within 100 days159.
The end of the infectious disease crisis can be accelerated through vaccine equity. The rise of resistant pathogens underscores the global failure to share vaccines equitably. Ensuring global recovery requires that the majority of the population, particularly the world’s poorest and most vulnerable, have access to sufficient vaccine doses. The temperature stability exhibited by some NVPCs46,118,160 could substantially contribute to efficient worldwide distribution by alleviating the challenges of cold-chain storage and transport.
While vaccines prevent infections before they occur, they don’t treat existing infections or reduce symptom severity and illness duration like antiviral drugs161. If NVPCs can fully replicate the viral entry process, they could serve as novel delivery platforms for drugs targeting virus-specific components to reduce infectivity and alleviate disease symptoms (Fig. 3). For instance, nucleic acid therapeutics could be used to interfere with the viral genome, while small molecules could inhibit key viral enzymes, such as reverse transcriptase or polymerase. Additionally, competing with the same host factors as the target virus to enter cells and release antiviral payloads could further enhance the efficacy of this strategy. However, to fully realize the potential of NVPCs in molecular delivery, several critical questions remain: do they bind to proteins in the bloodstream? How long do they circulate? Where do they accumulate? How immunogenic are they? Systematic in vivo experiments are essential to answer these questions. Understanding how factors such as size, shape, symmetry, and surface charge affect their behavior in the body will enable scientists to leverage the engineering toolbox at their disposal to continually improve NVPC design162.
By mimicking the early stages of the viral life cycle, NVPCs can be leveraged to develop antiviral strategies that both treat infected patients and prevent further viral spread. First, NVPCs compete for receptor binding, blocking viral entry. After cellular uptake via receptor-mediated endocytosis, engineered cages escape the endosome and release their payload (here depicted as an siRNA molecule). The delivery of nucleic acids targeting viral mRNA or host entry factors provides a final antiviral effect, significantly reducing viral infectivity. This figure was inspired by a template from BioRender176,177.
NVPCs are safe, malleable particles that resemble viruses and can be easily modified to mimic their functions, offering insights into viral virulence and enhancing therapeutic strategies against emerging threats. This perspective introduces NVPCs to an audience that may be unfamiliar with the technology, with the goal of inspiring cell biologists and virologists to incorporate them into their research. Additionally, the insights gained from studying viruses could benefit the protein engineering community, aiding in the development of NVPC-based delivery platforms to tackle other major health challenges. By fostering collaboration between these communities in cutting-edge multidisciplinary efforts, we can improve our ability to respond quickly to future pandemics and other global health crises.
Data availability
No datasets were generated or analysed during the current study.
References
Edwardson, T. G. W. & Hilvert, D. Virus-inspired function in engineered protein cages. J. Am. Chem. Soc. 141, 9432–9443 (2019).
Edwardson, T. G. W. et al. Protein cages: from fundamentals to advanced applications. Chem. Rev. 122, 9145–9197 (2022).
Li, L. et al. Binding and uptake of H-ferritin are mediated by human transferrin receptor-1. Proc. Natl. Acad. Sci. USA107, 3505–3510 (2010).
Green, M. & Loewenstein, P. M. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell 55, 1179–1188 (1988).
Nakase, I. et al. Cellular uptake of arginine-rich peptides: roles for macropinocytosis and actin rearrangement. Mol. Ther. 10, 1011–1022 (2004).
Kosuge, M., Takeuchi, T., Nakase, I., Jones, A. T. & Futaki, S. Cellular internalization and distribution of arginine-rich peptides as a function of extracellular peptide concentration, serum, and plasma membrane associated proteoglycans. Bioconjug. Chem. 19, 656–664 (2008).
Huang, S., Endo, R. I. & Nemerow, G. R. Upregulation of integrins alpha v beta 3 and alpha v beta 5 on human monocytes and T lymphocytes facilitates adenovirus-mediated gene delivery. J. Virol. 69, 2257–2263 (1995).
Pang, X. et al. Targeting integrin pathways: mechanisms and advances in therapy. Signal Transduct. Target. Ther. 8, 1 (2023).
Min, J., Kim, S., Lee, J. & Kang, S. Lumazine synthase protein cage nanoparticles as modular delivery platforms for targeted drug delivery. RSV Adv. 4, 48596–48600 (2014).
Kitagawa, T. et al. RGD-conjugated human ferritin nanoparticles for imaging vascular inflammation and angiogenesis in experimental carotid and aortic disease. Mol. Imag. Biol. 14, 315–324 (2012).
Zhen, Z. et al. RGD-modified apoferritin nanoparticles for efficient drug delivery to tumors. ACS Nano 7, 4830–4837 (2013).
Kitagawa, T. et al. RGD targeting of human ferritin iron oxide nanoparticles enhances in vivo MRI of vascular inflammation and angiogenesis in experimental carotid disease and abdominal aortic aneurysm. J. Magn. Reson. Imaging 45, 1144–1153 (2017).
Lee, Y., Kim, M., Kang, J. Y. & Jung, Y. Protein cages engineered for interaction-driven selective encapsulation of biomolecules. ACS Appl. Mater. Interfaces 14, 35357–35365 (2022).
Flenniken, M. L. et al. Melanoma and lymphocyte cell-specific targeting incorporated into a heat shock protein cage architecture. Chem. Biol. 13, 161–170 (2006).
Kang, H. J. et al. Developing an antibody-binding protein cage as a molecular recognition drug modular nanoplatform. Biomaterials 33, 5423–5430 (2012).
Falvo, E. et al. Antibody–drug conjugates: targeting melanoma with cisplatin encapsulated in protein-cage nanoparticles based on human ferritin. Nanoscale 5, 12278 (2013).
Lin, C.-Y., Yang, S.-J., Peng, C.-L. & Shieh, M.-J. Panitumumab-conjugated and platinum-cored pH-sensitive apoferritin nanocages for colorectal cancer-targeted therapy. ACS Appl. Mater. Interfaces 10, 6096–6106 (2018).
Hainfeld, J. F. Uranium-loaded apoferritin with antibodies attached: molecular design for uranium neutron-capture therapy. Proc. Natl. Acad. Sci. USA89, 11064–11068 (1992).
Fan, K. et al. Fenobody: a ferritin-displayed nanobody with high apparent affinity and half-life extension. Anal. Chem. 90, 5671–5677 (2018).
Kim, H. et al. Target-switchable Gd(III)-DOTA/protein cage nanoparticle conjugates with multiple targeting affibody molecules as target-selective T(1) contrast agents for high-field MRI. J. Control. Release 335, 269–280 (2021).
Choe, W., Durgannavar, T. A. & Chung, S. J. Fc-binding ligands of immunoglobulin G: an overview of high affinity proteins and peptides. Materials 9, 994 (2016).
Nilsson, B. et al. A synthetic IgG-binding domain based on staphylococcal protein a. Protein Eng. 1, 107 (1987).
Braisted, A. C. & Wells, J. A. Minimizing a binding domain from protein A. Proc. Natl. Acad. Sci. USA 93, 5688 (1996).
Ohno, K., Sawai, K., lijima, Y., Levin, B. & Meruelo, D. Cell-specific targeting of Sindbis virus vectors displaying IgG-binding domains of protein A. Nat. Biotechnol. 15, 763 (1997).
Ohno, K. & Meruelo, D. Retrovirus vectors displaying the IgG-binding domain of protein A. Biochem. Mol. Med. 62, 123–127 (1997).
Iijima, Y. et al. Cell-specific targeting of a thymidine kinase/ganciclovir gene therapy system using a recombinant Sindbis virus vector. Int. J. Cancer 80, 110–118 (1999).
Mottershead, D. G., Alfthan, K., Ojala, K., Takkinen, K. & Oker-Blom, C. Baculoviral display of functional scFv and synthetic IgG-binding domains. Biochem. Biophys. Res. Commun. 275, 84–90 (2000).
Ojala, K., Mottershead, D. G., Suokko, A. & Oker-Blom, C. Specific binding of baculoviruses displaying gp64 fusion proteins to mammalian cells. Biochem. Biophys. Res. Commun. 284, 777–784 (2001).
Morizono, K., Bristol, G., Xie, Y. -m, Kung, S. K.-P. & Chen, I. S. Y. Antibody-directed targeting of retroviral vectors via cell surface antigens. J. Virol. 75, 8016–8020 (2001).
Ried, M. U., Girod, A., Leike, K., Büning, H. & Hallek, M. Adeno-associated virus capsids displaying immunoglobulin-binding domains permit antibody-mediated vector retargeting to specific cell surface receptors. J. Virol. 76, 4559–4566 (2002).
Volpers, C. et al. Antibody-mediated targeting of an adenovirus vector modified to contain a synthetic immunoglobulin G-binding domain in the capsid. J. Virol. 77, 2093–2104 (2003).
Kim, H., Kang, Y. J., Min, J., Choi, H. & Kang, S. Development of an antibody-binding modular nanoplatform for antibody-guided targeted cell imaging and delivery. RSC Adv. 6, 19208–19213 (2016).
Levasseur, M. D. et al. Cell-specific delivery using an engineered protein nanocage. ACS Chem. Biol. 16, 838–843 (2021).
Ueda, G. et al. Tailored design of protein nanoparticle scaffolds for multivalent presentation of viral glycoprotein antigens. Elife 9, e57659 (2020).
Huotari, J. & Helenius, A. Endosome maturation. EMBO J. 30, 3481–3500 (2011).
Harrison, S. C. Viral membrane fusion. Nat. Struct. Mol. Biol. 15, 690–698 (2008).
Sriwilaijaroen, N. & Suzuki, Y. Molecular basis of the structure and function of H1 hemagglutinin of influenza virus. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 88, 226–249 (2012).
Yamauchi, Y. Influenza A virus uncoating. Adv. Virus Res. 106, 1–38 (2020).
Pinto, L. H., Holsinger, L. J. & Lamb, R. A. Influenza virus M2 protein has ion channel activity. Cell 69, 517–528 (1992).
Wharton, S. A., Belshe, R. B., Skehel, J. J. & Hay, A. J. Role of virion M2 protein in influenza virus uncoating: specific reduction in the rate of membrane fusion between virus and liposomes by amantadine. J. Gen. Virol. 75, 945–948 (1994).
Staring, J., Raaben, M. & Brummelkamp, T. R. Viral escape from endosomes and host detection at a glance. J. Cell Sci. 131, jcs216259 (2018).
Shen, H. et al. De novo design of pH-responsive self-assembling helical protein filaments. Nat. Nanotechnol. 19, 1016–1021 (2024).
King, N. P. et al. Computational design of self-assembling protein nanomaterials with atomic level accuracy. Science 336, 1171–1174 (2012).
Edwardson, T. G. W., Mori, T. & Hilvert, D. Rational engineering of a designed protein cage for siRNA delivery. J. Am. Chem. Soc. 140, 10439–10442 (2018).
Edwardson, T. G. W., Tetter, S. & Hilvert, D. Two-tier supramolecular encapsulation of small molecules in a protein cage. Nat. Commun. 11, 5410 (2020).
Edwardson, T. G. W., Levasseur, M. D. & Hilvert, D. The OP protein cage: a versatile molecular delivery platform. Chimia 75, 323–328 (2021).
Behr, J.-P. The Proton sponge: a trick to enter cells the viruses did not exploit. Chimia 51, 34 (1997).
Pichon, C., Goncalves, C. & Midoux, P. Histidine-rich peptides and polymers for nucleic acids delivery. Adv. Drug Deliv. Rev. 53, 75–94 (2001).
Belyi, V. A. & Muthukumar, M. Electrostatic origin of the genome packing in viruses. Proc. Natl. Acad. Sci. USA103, 17174–17178 (2006).
Lilavivat, S., Sardar, D., Jana, S., Thomas, G. C. & Woycechowsky, K. J. In vivo encapsulation of nucleic acids using an engineered nonviral protein capsid. J. Am. Chem. Soc. 134, 13152–13155 (2012).
Azuma, Y., Edwardson, T. G. W., Terasaka, N. & Hilvert, D. Modular protein cages for size-selective RNA packaging in vivo. J. Am. Chem. Soc. 140, 566–569 (2018).
Butterfield, G. L. et al. Evolution of a designed protein assembly encapsulating its own RNA genome. Nature 552, 415–420 (2017).
Azuma, Y., Herger, M. & Hilvert, D. Diversification of protein cage structure using circularly permuted subunits. J. Am. Chem. Soc. 140, 558–561 (2018).
Terasaka, N., Azuma, Y. & Hilvert, D. Laboratory evolution of virus-like nucleocapsids from nonviral protein cages. Proc. Natl. Acad. Sci. USA115, 5432–5437 (2018).
Tetter, S. et al. Evolution of a virus-like architecture and packaging mechanism in a repurposed bacterial protein. Science 372, 1220–1224 (2021).
Qu, C. et al. 3D domain swapping modulates the stability of members of an icosahedral virus group. Structure 8, 1095–1103 (2000).
Sun, Z. et al. Double-stranded RNA virus outer shell assembly by bona fide domain-swapping. Nat. Commun. 8, 14814 (2017).
Krupovic, M., Dolja, V. V. & Koonin, E. V. Origin of viruses: primordial replicators recruiting capsids from hosts. Nat. Rev. Microbiol. 17, 449–458 (2019).
Olshefsky, A. & King, N. P. Hallmarks of icosahedral virus capsids emerged during laboratory evolution of a bacterial enzyme. Trends Biochem. Sci. 46, 863–865 (2021).
Steel, J. & Lowen, A. C. Influenza A virus reassortment. Curr. Top. Microbiol. Immunol. 385, 377–401 (2014).
Ganti, K. et al. Influenza A virus reassortment in mammals gives rise to genetically distinct within-host subpopulations. Nat. Commun. 13, 6846 (2022).
Smith, G. J. et al. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature 459, 1122–1125 (2009).
Herpoldt, K. L. et al. Macromolecular cargo encapsulation via in vitro assembly of two-component protein nanoparticles. Adv. Healthc. Mater. 13, e2303910 (2024).
Hori, M. et al. Stimulus-responsive assembly of nonviral nucleocapsids. Nat. Commun. 15, 3576 (2024).
Azizgolshani, O., Garmann, R. F., Cadena-Nava, R., Knobler, C. M. & Gelbart, W. M. Reconstituted plant viral capsids can release genes to mammalian cells. Virology 441, 12–17 (2013).
Comas-Garcia, M. et al. Characterization of viral capsid protein self-assembly around short single-stranded RNA. J. Phys. Chem. B 118, 7510–7519 (2014).
Singaram, S. W., Garmann, R. F., Knobler, C. M., Gelbart, W. M. & Ben-Shaul, A. Role of RNA branchedness in the competition for viral capsid proteins. J. Phys. Chem. B 119, 13991–14002 (2015).
Borodavka, A. et al. Sizes of long RNA molecules are determined by the branching patterns of their secondary structures. Biophys J 111, 2077–2085 (2016).
Beren, C., Dreesens, L. L., Liu, K. N., Knobler, C. M. & Gelbart, W. M. The effect of RNA secondary structure on the self-assembly of viral capsids. Biophys. J. 113, 339–347 (2017).
Knobler, C. M. & Gelbart, W. M. How and why RNA genomes are (partially) ordered in viral capsids. Curr. Opin. Virol. 52, 203–210 (2022).
Alston, J. J. & Soranno, A. Condensation goes viral: a polymer physics perspective. J. Mol. Biol. 435, 167988 (2023).
Alenquer, M. et al. Influenza A virus ribonucleoproteins form liquid organelles at endoplasmic reticulum exit sites. Nat. Commun. 10, 1629 (2019).
Etibor, T. A., Yamauchi, Y. & Amorim, M. J. Liquid biomolecular condensates and viral lifecycles: review and perspectives. Viruses 13, 366 (2021).
Etibor, T. A. et al. Defining basic rules for hardening influenza A virus liquid condensates. Elife 12, e85182 (2023).
Etibor, T. A. et al. Challenges in imaging analyses of biomolecular condensates in cells infected with influenza A virus. Int. J. Mol. Sci. 24, 15253 (2023).
Oh, H. J. et al. Size-controlled assembly of phase separated protein condensates with interfacial protein cages. Nat. Commun. 16, 1009 (2025).
Payne, S. in Viruses 25–37 (Academic Press, 2023).
Sundquist, W. I. & Krausslich, H. G. HIV-1 assembly, budding, and maturation. Cold Spring Harb. Perspect. Med. 2, a006924 (2012).
Freed, E. O. HIV-1 gag proteins: diverse functions in the virus life cycle. Virology 251, 1–15 (1998).
Ono, A., Ablan, S. D., Lockett, S. J., Nagashima, K. & Freed, E. O. Phosphatidylinositol (4,5) bisphosphate regulates HIV-1 Gag targeting to the plasma membrane. Proc. Natl. Acad. Sci. USA101, 14889–14894 (2004).
Votteler, J. & Sundquist, W. I. Virus budding and the ESCRT pathway. Cell Host Microbe 14, 232–241 (2013).
Hsia, Y. et al. Design of a hyperstable 60-subunit protein icosahedron. Nature 535, 136 (2016).
Votteler, J. et al. Designed proteins induce the formation of nanocage-containing extracellular vesicles. Nature 540, 292–295 (2016).
Zhao, G. et al. Mature HIV-1 capsid structure by cryo-electron microscopy and all-atom molecular dynamics. Nature 497, 643–646 (2013).
Quemin, E. R. J. et al. Cellular electron cryo-tomography to study virus-host interactions. Annu. Rev. Virol. 7, 239–262 (2020).
Graham, M. & Zhang, P. Cryo-electron tomography to study viral infection. Biochem. Soc. Trans. 51, 1701–1711 (2023).
Wang, Y. et al. Pseudotyped viruses. Adv. Exp. Med. Biol. 1407, 1–27 (2023).
Cronin, J., Zhang, X. Y. & Reiser, J. Altering the tropism of lentiviral vectors through pseudotyping. Curr. Gene Ther. 5, 387–398 (2005).
Li, Q., Liu, Q., Huang, W., Li, X. & Wang, Y. Current status on the development of pseudoviruses for enveloped viruses. Rev. Med. Virol. 28, e1963 (2018).
Tortorella, D., Gewurz, B. E., Furman, M. H., Schust, D. J. & Ploegh, H. L. Viral subversion of the immune system. Annu. Rev. Immunol. 18, 861–926 (2000).
Alcami, A. & Koszinowski, U. H. Viral mechanisms of immune evasion. Trends Microbiol. 8, 410–418 (2000).
Wyatt, R. et al. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature 393, 705–711 (1998).
Wei, X. et al. Antibody neutralization and escape by HIV-1. Nature 422, 307–312 (2003).
Watanabe, Y., Allen, J. D., Wrapp, D., McLellan, J. S. & Crispin, M. Site-specific glycan analysis of the SARS-CoV-2 spike. Science 395, eabb9983 (2020).
Tytgat, H. L. P. et al. Cytoplasmic glycoengineering enables biosynthesis of nanoscale glycoprotein assemblies. Nat. Commun. 10, 5403 (2019).
Keys, T. G. et al. A biosynthetic route for polysialylating proteins in Escherichia coli. Metab. Eng. 44, 293–301 (2017).
Kightlinger, W. et al. A cell-free biosynthesis platform for modular construction of protein glycosylation pathways. Nat. Commun. 10, 5404 (2019).
Hershewe, J., Kightlinger, W. & Jewett, M. C. Cell-free systems for accelerating glycoprotein expression and biomanufacturing. J. Ind. Microbiol. Biotechnol. 47, 977–991 (2020).
Minor, P. D. Live attenuated vaccines: historical successes and current challenges. Virology 479-480, 379–392 (2015).
Cramer, J. P. In Travel Medicine 65–73 https://www.sciencedirect.com/science/article/abs/pii/B9780323546966000094 (Elsevier, 2019).
Zhao, T. et al. Vaccine adjuvants: mechanisms and platforms. Signal Transduct. Target. Ther. 8, 283 (2023).
Nguyen, B. & Tolia, N. H. Protein-based antigen presentation platforms for nanoparticle vaccines. npj Vaccines 6, 70 (2021).
Bachmann, M. F. & Jennings, G. T. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 10, 787–796 (2010).
Ahmadivand, S., Fux, R. & Palic, D. Ferritin vaccine platform for animal and zoonotic viruses. Vaccines 12, 1112 (2024).
Ladenstein, R. & Morgunova, E. Second career of a biosynthetic enzyme: lumazine synthase as a virus-like nanoparticle in vaccine development. Biotechnol. Rep. 27, e00494 (2020).
Rappuoli, R. & Serruto, D. Self-assembling nanoparticles usher in a new era of vaccine design. Cell 176, 1245–1247 (2019).
Ellis, D. et al. Antigen spacing on protein nanoparticles influences antibody responses to vaccination. Cell Rep. 42, 113552 (2023).
Papazisis, G. et al. Respiratory syncytial virus vaccines: analysis of pre-marketing clinical trials for immunogenicity in the population over 50 years of age. Vaccines 12, 353 (2024).
Boyoglu-Barnum, S. et al. Quadrivalent influenza nanoparticle vaccines induce broad protection. Nature 592, 623–628 (2021).
Cohen, A. A. et al. Mosaic nanoparticles elicit cross-reactive immune responses to zoonotic coronaviruses in mice. Science 371, 735–741 (2021).
Chao, C. W. et al. Protein nanoparticle vaccines induce potent neutralizing antibody responses against MERS-CoV. Cell Rep. 43, 115036 (2024).
Wang, E. et al. Designed mosaic nanoparticles enhance cross-reactive immune responses in mice. Cell 188, 1036–1050 (2025).
Hills, R. A. et al. Proactive vaccination using multiviral Quartet Nanocages to elicit broad anti-coronavirus responses. Nat. Nanotechnol. 19, 1216–1223 (2024).
Cohen, A. A. et al. Mosaic sarbecovirus nanoparticles elicit cross-reactive responses in pre-vaccinated animals. Cell 187, 5554–5571.e5519 (2024).
Marcandalli, J. et al. Induction of Potent neutralizing antibody responses by a designed protein nanoparticle vaccine for respiratory syncytial virus. Cell 176, 1420–1431.e1417 (2019).
Brouwer, P. J. M. et al. Two-component spike nanoparticle vaccine protects macaques from SARS-CoV-2 infection. Cell 184, 1188–1200.e1119 (2021).
Walls, A. C. et al. Elicitation of potent neutralizing antibody responses by designed protein nanoparticle vaccines for SARS-CoV-2. Cell 183, 1367–1382.e1317 (2020).
Antanasijevic, A. et al. Structural and functional evaluation of de novo-designed, two-component nanoparticle carriers for HIV Env trimer immunogens. PLoS Pathog. 16, e1008665 (2020).
Lacasta, A. et al. Design and immunological evaluation of two-component protein nanoparticle vaccines for East Coast fever. Front. Immunol. 13, 1015840 (2022).
Lee, S. et al. Four-component protein nanocages designed by programmed symmetry breaking. Nature 638, 546–552 (2025).
Dadonaite, B. et al. Deep mutational scanning of H5 hemagglutinin to inform influenza virus surveillance. PLoS Biol. 22, e3002916 (2024).
Olshefsky, A. et al. In vivo selection of synthetic nucleocapsids for tissue targeting. Proc. Natl. Acad. Sci. USA 120, e2306129120 (2023).
Carroll, D. et al. Preventing the next pandemic: the power of a global viral surveillance network. BMJ 372, n485 (2021).
Balistreri, G., Yamauchi, Y. & Teesalu, T. A widespread viral entry mechanism: the C-end Rule motif-neuropilin receptor interaction. Proc. Natl. Acad. Sci. USA 118, e2112457118 (2021).
Millet, J. K. & Whittaker, G. R. Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein. Proc. Natl. Acad. Sci. USA111, 15214–15219 (2014).
Park, J. E. et al. Proteolytic processing of Middle East respiratory syndrome coronavirus spikes expands virus tropism. Proc. Natl. Acad. Sci. USA 113, 12262–12267 (2016).
Hoffmann, M., Kleine-Weber, H. & Pohlmann, S. A multibasic cleavage site in the spike protein of SARS-CoV-2 Is essential for infection of human lung cells. Mol. Cell. 78, 779–784.e775 (2020).
Menachery, V. D. et al. Trypsin treatment unlocks barrier for zoonotic bat coronavirus infection. J. Virol. 94, e01774–19 (2020).
Peacock, T. P. et al. The furin cleavage site in the SARS-CoV-2 spike protein is required for transmission in ferrets. Nat. Microbiol. 6, 899–909 (2021).
Bosch, F. X., Orlich, M., Klenk, H. D. & Rott, R. The structure of the hemagglutinin, a determinant for the pathogenicity of influenza viruses. Virology 95, 197–207 (1979).
Ito, T. et al. Generation of a highly pathogenic avian influenza A virus from an avirulent field isolate by passaging in chickens. J. Virol. 75, 4439–4443 (2001).
Horimoto, T. & Kawaoka, Y. Reverse genetics provides direct evidence for a correlation of hemagglutinin cleavability and virulence of an avian influenza A virus. J. Virol. 68, 3120–3128 (1994).
Swayne, D. E. Understanding the complex pathobiology of high pathogenicity avian influenza viruses in birds. Avian Dis. 51, 242–249 (2007).
Monne, I. et al. Emergence of a highly pathogenic avian influenza virus from a low-pathogenic progenitor. J. Virol. 88, 4375–4388 (2014).
Abdelwhab, E. M. et al. Composition of the hemagglutinin polybasic proteolytic cleavage motif mediates variable virulence of H7N7 avian influenza viruses. Sci. Rep. 6, 39505 (2016).
Teesalu, T., Sugahara, K. N., Kotamraju, V. R. & Ruoslahti, E. C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proc. Natl. Acad. Sci. USA 106, 16157–16162 (2009).
Kolodkin, A. L. et al. Neuropilin is a semaphorin III receptor. Cell 90, 753–762 (1997).
Ellis, L. M. The role of neuropilins in cancer. Mol. Cancer Ther. 5, 1099–1107 (2006).
Glinka, Y. & Prud’homme, G. J. Neuropilin-1 is a receptor for transforming growth factor beta-1, activates its latent form, and promotes regulatory T cell activity. J. Leukoc. Biol. 84, 302–310 (2008).
Wang, H.-B. et al. Neuropilin 1 is an entry factor that promotes EBV infection of nasopharyngeal epithelial cells. Nat. Commun. 6, 6240 (2015).
Lu, Z. Z. et al. Neuropilin 1 is an entry receptor for KSHV infection of mesenchymal stem cell through TGFBR1/2-mediated macropinocytosis. Sci. Adv. 9, eadg1778 (2023).
Shang, P. et al. NRP1 is a receptor for mammalian orthoreovirus engaged by distinct capsid subunits. Cell Host Microbe 32, 980–995.e989 (2024).
Yu, H. et al. Neuropilin-1 is a novel host factor modulating the entry of hepatitis B virus. J. Hepatol. 82, 37–50 (2025).
Cantuti-Castelvetri, L. et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 370, 856–860 (2020).
Daly, J. L. et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science 370, 861–865 (2020).
Levasseur, M. D. et al. Post-assembly modification of protein cages by Ubc9-mediated lysine acylation. ChemBioChem 23, e202200332 (2022).
Kaster, M. A. et al. Engineered nonviral protein cages modified for MR imaging. ACS Appl. Bio Mater. 6, 591–602 (2023).
Seebeck, F. P., Woycechowsky, K. J., Zhuang, W., Rabe, J. P. & Hilvert, D. A simple tagging system for protein encapsulation. J. Am. Chem. Soc. 128, 4516–4517 (2006).
Brasko, C. et al. Intelligent image-based in situ single-cell isolation. Nat. Commun. 9, 226 (2018).
Lundberg, E. & Borner, G. H. H. Spatial proteomics: a powerful discovery tool for cell biology. Nat. Rev. Mol. Cell Biol. 20, 285–302 (2019).
Mund, A. et al. Deep visual proteomics defines single-cell identity and heterogeneity. Nat. Biotechnol. 40, 1231–1240 (2022).
Rennick, J. J., Johnston, A. P. R. & Parton, R. G. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotechnol. 16, 266–276 (2021).
Yeates, T. O., Agdanowski, M. P. & Liu, Y. Development of imaging scaffolds for cryo-electron microscopy. Curr. Opin. Struct. Biol. 60, 142–149 (2020).
Castells-Graells, R. et al. Cryo-EM structure determination of small therapeutic protein targets at 3 Å-resolution using a rigid imaging scaffold. Proc. Natl. Acad. Sci. USA 120, e2305494120 (2023).
Hassell, J. M., Begon, M., Ward, M. J. & Fevre, E. M. Urbanization and disease emergence: dynamics at the wildlife-livestock-human interface. Trends Ecol. Evol. 32, 55–67 (2017).
Carlson, C. J. et al. Climate change increases cross-species viral transmission risk. Nature 607, 555–562 (2022).
Rupasinghe, R., Chomel, B. B. & Martinez-Lopez, B. Climate change and zoonoses: a review of the current status, knowledge gaps, and future trends. Acta Trop. 226, 106225 (2022).
Eby, P. et al. Pathogen spillover driven by rapid changes in bat ecology. Nature 613, 340–344 (2023).
CEPI. CEPI 2.0 and the 100 Days Mission. https://cepi.net/cepi-20-and-100-days-mission.
Azuma, Y., Edwardson, T. G. W. & Hilvert, D. Tailoring lumazine synthase assemblies for bionanotechnology. Chem. Soc. Rev. 47, 3543–3557 (2018).
von Delft, A. et al. Accelerating antiviral drug discovery: lessons from COVID-19. Nat. Rev. Drug Discov. 22, 585–603 (2023).
Olshefsky, A., Richardson, C., Pun, S. H. & King, N. P. Engineering self-assembling protein nanoparticles for therapeutic delivery. Bioconjugate Chem. 33, 2018–2034 (2022).
Dimitrov, D. S. Virus entry: molecular mechanisms and biomedical applications. Nat. Rev. Microbiol. 2, 109–122 (2004).
Villanueva, R. A., Rouille, Y. & Dubuisson, J. Interactions between virus proteins and host cell membranes during the viral life cycle. Int. Rev. Cytol. 245, 171–244 (2005).
Yamauchi, Y. & Helenius, A. Virus entry at a glance. J. Cell Sci. 126, 1289–1295 (2013).
Helenius, A. Virus entry: looking back and moving forward. J. Mol. Biol. 430, 1853–1862 (2018).
Wörsdörfer, B., Woycechowsky, K. J. & Hilvert, D. Directed evolution of a protein container. Science 331, 589–592 (2011).
Beck, T., Tetter, S., Künzle, M. & Hilvert, D. Construction of Matryoshka-type structures from supercharged protein nanocages. Angew Chem. Int. Ed. 54, 937–940 (2015).
Bruun, T. U. J., Andersson, A.-M. C., Draper, S. J. & Howarth, M. Engineering a rugged nanoscaffold to enhance plug-and-display vaccination. ACS Nano 12, 8855–8866 (2018).
Zakeri, B. et al. Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Proc. Natl. Acad. Sci. USA 109, E690–E697 (2012).
Bale, J. B. et al. Accurate design of megadalton-scale two-component icosahedral protein complexes. Science 353, 389–394 (2016).
Yang, E. C. et al. Computational design of non-porous pH-responsive antibody nanoparticles. Nat. Struct. Mol. Biol. 31, 1404–1412 (2024).
Meng, E. C. et al. UCSF ChimeraX: tools for structure building and analysis. Protein Sci. 32, e4792(2023).
Pettersen, E. F. et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 30, 70–82 (2021).
Turner, J. et al. EMDB—the electron microscopy data bank. Nucleic Acids Res. 52, D456–D465 (2024).
BioRender. Novel nanoparticles target oncogenic long non-coding RNA for cancer therapy. https://app.biorender.com/biorender-templates/t-5fc94bce030bd3328427935b-novel-nanoparticles-target-oncogenic-long-non-coding-rna-for (2020).
Vaidya, A. M. et al. Systemic delivery of tumor-targeting siRNA nanoparticles against an oncogenic LncRNA facilitates effective triple-negative breast cancer therapy. Bioconjug. Chem. 30, 907–919 (2019).
Acknowledgements
I thank members of the Molecular Medicine Group, Prof. Donald Hilvert and Dr. Thomas Edwardson at ETH Zürich for helpful comments and discussion. Generous support from Prof. Peter Kast and ETH Zürich is also gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
M.D.L. wrote the manuscript text and prepared the figures.
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Levasseur, M.D. Nonviral protein cages as tools to decipher and combat viral threats. npj Viruses 3, 45 (2025). https://doi.org/10.1038/s44298-025-00127-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s44298-025-00127-8
This article is cited by
-
Harnessing Nanocarriers to Advance Vaccine Development
BioDrugs (2026)