Abstract
Intracoronary stents have revolutionized the treatment of coronary obstructions, allowing for minimally invasive procedures and significantly enhancing both therapeutic efficacy and patient quality of life. Despite their clinical success, once implanted, stents act as passive elements, incapable of providing real-time data on the treated artery, thereby limiting early diagnosis of post-implantation complications such as inflammation, restenosis, or recurrence of atheromatous plaques. In this study, we introduce stents with deep tissue sensing capabilities through the incorporation of near-infrared emitting lanthanide-doped nanoparticles. Using 3D printed stent prototypes, we demonstrate that the luminescence emitted by neodymium and ytterbium ions in core/shell/shell NaYF₄ nanoparticles facilitates both deep tissue visualization and tracking of the stent position. Additionally, the temperature-dependent fluorescence lifetime of Nd,Yb:NaYF₄ nanoparticles provides thermal sensitivity, allowing precise deep tissue thermal monitoring of catheter-induced heating with sub-degree accuracy. This work highlights the potential of infrared-emitting nanoparticles to drive the next generation of coronary stents, paving the way for advanced light-based diagnostics and therapeutic procedures.
Similar content being viewed by others
Introduction
Coronary implants (stents) have deeply transformed the treatment of cardiovascular diseases, especially in the revascularization of obstructed coronary arteries, significantly improving the survival and quality of life of millions of patients worldwide1. These implants are essential for maintaining arterial functionality. The positive results obtained in comparison with surgery-assisted, artery reconstruction have consolidated stents as a cornerstone in interventional cardiology. Furthermore, coating the surface of stents with polymers and drugs has significantly minimized post-implantation complications. For instance, the use of drug-eluting stents instead of bare metallic stents has substantially lowered the risk of restenosis, offering patients a more durable solution with fewer adverse outcomes2. However, despite their effectiveness, current stents are passive elements which are unable to provide real-time diagnostic data: once implanted, they cannot give insight into the local physiological conditions of the surrounding tissue or the status of the device. This limitation prevents reaching an accurate diagnosis of the damaged artery. For instance, real-time monitoring of stent (artery) temperature would allow for early diagnosis of inflammation or detection of the development of plaque clots at early stages. The combination of integrated electronics into stents has been proposed in recent years as a possible solution to achieve post-implantation diagnosis of intervened arteries3,4. Nevertheless, the integration of emitting electronics into stents is technologically complex and could impact the final cost of the whole surgical process. Therefore, there is an unmet need to develop alternative routes to provide stents with diagnostic capabilities. Among these alternatives, the combination of stents with luminescent sensors can be a promising approach.
The fabrication of multifunctional implants incorporating luminescent probes has been demonstrated in multiple cases5,6,7,8,9,10,11,12,13,14,15,16,17 (see Supplementary Table S1 in Supplementary Information), including applications such as bioimaging, in situ monitoring of bone regeneration progression, glucose detection and photodynamic therapy. However, the potential of such an approach in stent-based applications remains to be explored.
When selecting optical probes to provide extra functionality to coronary stents, optimal candidates must satisfy the following requisites:
-
i.
Present negligible toxicity in a cardiovascular environment,
-
ii.
Be robust enough to retain their luminescent properties during the implant fabrication process,
-
iii.
Operate in one of the biological windows to allow for deep tissue imaging.
-
iv.
They must be capable of remote sensing of any of the physiological parameters of relevance for intracoronary diagnosis such as temperature, oxygen concentration, or inflammatory markers.
Lanthanide doped nanoparticles (Ln:NPs) simultaneously fulfill all of the requisites described above: Ln:NPs have been widely used for in vivo applications revealing minimal cytotoxicity5, they have already been used for plaque imaging18, they can operate in the second biological window (II-BW, 1000–1400 nm) achieving large penetration into tissues19,20,21, and they are considered robust probes against degradation. The incorporation of “conventional” II-BW emitting Ln:NPs into stents would allow their deep tissue visualization and tracking without the need to use ionizing radiation, such as X-rays. In addition, some II-BW NPs show luminescence properties which are strongly dependent on the physiological conditions (such as pH, H2O2 or temperature) providing the stent with sensing potential22. Of special relevance is the capability of some II-BW emitting Ln:NPs to work as remote thermal sensors22,23,24. Temperature is an early sign of inflammatory processes and infections, as well as an indicator of the presence of incipient atheroma plaques25,26,27,28,29,30. Furthermore, remote sensing of intracoronary temperature would provide feedback during thermal treatments for thrombosis and unstable atheroma plaque28,31. Thus, the inclusion of II-BW emitting Ln:NPs within stents would transform them from passive to active implants capable of imaging and diagnosis. Despite its interest, this possibility has not yet been explored.
In this study, we present the first application of a luminescent vascular stent for thermometry and investigate the potential of II-BW emitting Ln:NPs to confer additional functionalities to cardiovascular implants (stents). We report on the controlled incorporation of II-BW emitting neodymium and ytterbium co-doped nanoparticles during the 3D printing of a stent prototype. The luminescence properties and thermal sensing capabilities of the II-BW emitting Ln:NPs are investigated before and after the 3D printing process. Furthermore, we design and perform proof-of-concept experiments to demonstrate the potential of the luminescent stents for sub-tissue localization and tracking as well as for real-time thermal monitoring during catheter-assisted intracoronary procedures.
Results
Fabrication and characterization of 3D printed luminescent stents
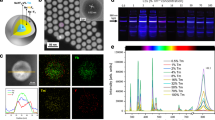
Luminescent 3D printed stents are fabricated by using ytterbium and neodymium co-doped NaYF4 NPs (Nd, Yb:NPs) as luminescent building blocks. These Nd,Yb:NPs are constituted by an inert NaYF4 core, an active neodymium and ytterbium co-doped NaYF4 shell, and a second undoped shell of CaF2. This structure and doping distribution (Fig. 1a) used were employed previously, as they simultaneously resulted in a high brightness, high thermal sensitivity and high chemical stability32,33. A representative Transmission Electron Microscopy (TEM) image of Ln:NPs reveals an average size of 12 ± 3 nm (Fig. 1b and Supplementary Fig. S1 in Supplementary Information). Under 800 nm optical excitation (4I9/2 → 4F5/2 neodymium excitation), Ln:NPs show an emission band extending from 950 up to 1100 nm with a predominant emission at 980 nm (Fig. 1c). This emission band is associated to the 2F5/2 → 2F7/2 radiative decay of ytterbium ions that are being excited thanks to the effective neodymium to ytterbium nonradiative energy transfer34. The emission of Nd,Yb:NPs lies within the II-BW allowing for sub-tissue imaging and sensing, as has been demonstrated by recent studies dealing with in vivo imaging and thermal sensing in animal models35. For the fabrication of luminescent stents, the Nd,Yb:NPs are dispersed into a photosensitive, acrylic-based resin which is then used to 3D print the implant (Fig. 1d). We reproduce the structure of coronary stents with a radius of 2 mm and a total length of 1.5 cm, mimicking the dimensions of a real stent after the implantation procedure (see optical picture in Fig. 1e). Uniaxial compression tests of the 3D printed stents revealed that the incorporation of Nd,Yb:NPs results in a reduction in mechanical stiffness by approximately 63% (Supplementary Fig. S2 in Supplementary Information). The underlying mechanism responsible for this nanoparticle-induced decrease in stiffness remains unclear at this stage but will be investigated in the future, given the critical importance of mechanical properties in the performance of coronary implants36. Under 800 nm optical excitation, the fabricated stents show a strong II-BW infrared emission (Fig. 1f) that reveals an efficient and homogeneous distribution of Nd,Yb:NPs within the structure. Further, the measured emission spectra from the stents are virtually identical to those of the Nd,Yb:NPs suspended in hexane (Fig. 1c). In addition, the fluorescence decay curves (λexc = 800 nm, λem = 980 nm) corresponding to Nd,Yb:NPs in the hexane solution and within the stent show only slight differences that can be correlated to the different interactions between the Nd,Yb:NPs and the surrounding (hexane or polymer) molecules (Fig. 1g). Figure 1c, g point out that the spectroscopic properties of Nd,Yb:NPs do not deteriorate during the 3D printing process. This can be attributed, among other factors, to the presence of the CaF2 shell that protects the emitting/active volume of our Ln:NPs from a possible degradation caused by the interaction with the photosensitive resin used for 3D printing. We also note that the spectral operation range of the fabricated stents can be tuned by changing the lanthanide ions incorporated in the NaYF4 nanoparticles. We have fabricated similar stents using Er,Yb co-doped nanoparticles, resulting in fluorescent stent operating at 1.5 µm, i.e. in the third biological window (see Supplementary Fig. S3 in Supplementary Information)37,38. The potential of stent-induced toxicity was evaluated using MTT assays on mouse aortic endothelial cell. Results (Fig. 1h) reveal a negligible impact of the Nd,Yb:NPs+polymer system on the viability of endothelial cells. The absence of toxicity agrees with previous toxicity studies in mice that revealed long-term administration of lanthanide-doped nanoparticles in mice did not lead to any noticeable toxic effects39. Our findings as well as previous works offer evidence regarding the safety of implants containing lanthanide-doped nanoparticles.
Schematic drawing (a) and representative TEM image (b) of the core/shell/shell structure of the neodymium and ytterbium co-doped NaYF4 nanoparticles used in this work to provide extra functionalities to 3D printed stents. c Room temperature emission of the neodymium and ytterbium co-doped nanoparticles obtained when dispersed in hexane and after incorporation into the stent under 800 nm excitation. d Schematic representation of the 3D printing procedure. Optical (e) and II-BW fluorescence (f) image of the 3D printed stent. For the NIR-II image, the stent was illuminated with an 800 nm laser and the image was collected in the 900–1200 nm spectral range. g Room temperature decay curves corresponding to the neodymium and ytterbium co-doped nanoparticles obtained when dispersed in hexane and after incorporation into the stent after the 3D printing process. Data obtained after optical excitation with a 8 ns pulsed laser operating at 800 nm and by collecting the emitted radiation at 980 nm. h Relative cell viability of mouse aortic endothelial cells incubated with different doses of stent powder for 24 h, as analyzed by the MTT method (five independent repeats (n = 5)).
Sub-tissue imaging capabilities of the 3D printed luminescent stents
The potential use of the 3D printed luminescent stent for intracoronary localization and tracking is explored by a simple proof-of-concept experiment (Fig. 2a). The luminescent stent is inserted into a plastic tube (4.6 mm in diameter), mimicking a coronary artery. The whole system (“tube + stent”) is covered with chicken breast tissue of variable thickness. The tissue was then illuminated with an expanded 800 nm laser beam providing an on-target power density of 140 mW/cm2, well below the tissue damage level40. As the tissue thickness increases, the contrast and resolution of the fluorescence image decrease as expected due to the absorption and scattering of both the excitation and emitted radiation41. The fluorescence images collected by an InGaAs camera reveal that locating the stent is possible for tissue thicknesses up to 4 cm (Fig. 2b). The detected NP fluorescence intensity (\({I}_{\det }\)) stems from the combined absorption and scattering of both excitation light (800 nm) and the emitted fluorescence (980 nm) traveling into and out of the tissue, respectively. Rather than exhaustively describing this complex system, given the NP fluorescence decreases exponentially with the tissue thickness (\({d}_{t}\)), we employ the following phenomenological model:
where \({I}_{0}\) is the intensity detected in absence of any tissue and \({l}_{{eff}}\) is the effective penetration depth. The fit of experimental data (Fig. 2c) to Eq. (1) leads to an effective penetration depth of 0.85 cm, that agrees with results previously reported in literature35. Further improvement of this imaging depth could be easily achieved using higher 800 nm excitation power densities (not available in our experimental set-up) and, above all, using infrared fluorescence cameras of higher sensitives and reduced background noise. The large penetration depth of the II-BW luminescence achieved using our 3D printed luminescent stents does not only allow for localization but also opens the possibility of dynamically tracking the stent’s position within the arteries. To demonstrate this possibility, we simulated the motion of a stent-detaching event caused by incomplete stent apposition (one of the most risky situations during stent implantation in clinical practice)42. The stent’s position was changed by pushing the stent with a catheter while recording a fluorescence video (see Fig. 2d and the Supplementary Video S1 in Supplementary Section S5 of Supplementary Information). The sequence of fluorescence images evidence that it is possible to track the position of the stent behind the tissue (Fig. 2e) and, hence, detect in advance a possible failure in stent implantation. To assess the impact of a physiological environment on the luminescence properties of the stent, we incubated the stents in fetal bovine serum (FBS) at 37 °C for five days. Under these conditions, the formation of a protein coating is expected, which could potentially influence the fluorescence characteristics of the stent. However, after five days of incubation, no significant degradation in luminescence performance was observed. The emission spectral profile, integrated fluorescence intensity, and fluorescence lifetime all remained essentially unchanged, indicating that the optical properties of the stent are well preserved under physiologically relevant conditions (see Supplementary Fig. S4 and Supplementary Section S6 in Supplementary Information).
a Schematic drawing of the experimental procedure used to evaluate the sub-tissue imaging capabilities of the 3D printed luminescent stents. b Fluorescence images of the luminescent stent and c Integrated luminescence intensity corresponding to the fluorescence images obtained under different tissue thicknesses, performed once (n = 1). Scatter points represent experimental data, and line is the best fit to Eq. (1). d Schematic representation of the experimental design, demonstrating the potential of our 3D printed luminescent stent for dynamical tracking during a detaching event. e Fluorescence images of a moving stent obtained under a 0.5 cm thick tissue. All scale bars in the figures represent 5 cm.
Lifetime-based sensing capability of 3D printed luminescent stent
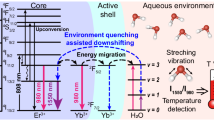
The Nd,Yb:NPs incorporated into the stent have also been used in the past for thermal sensing based on the strong temperature dependence of their fluorescence lifetime35. Retaining this property during the 3D printing process, the stents are then endowed with thermal sensing capabilities, enabling real-time monitoring of temperature variations within the tissue. To corroborate the relative thermal sensitivity of the 3D printed stent, we placed the stent within a tube and modified its temperature by controlling heating of the water circulating within the tube (see Fig. 3a). An 800 nm, 8 ns pulsed laser was used to optically excite the stent through the tissue. The fluorescence emitted at 980 nm was detected by a NIR-II photomultiplier and the luminescence decay curve was recorded using a digital oscilloscope. The decay curves are found to be non-exponential in the whole temperature range investigated in this work (Fig. 3b). This is in accordance with previous works and is due, mainly, to the presence of nonradiative energy transfer processes. In addition, the decay curves become faster as the temperature is increased due to the enhancement in the phonon-assisted Nd³⁺→Yb³⁺ energy transfer (Fig. 3b)34. Upon excitation at 800 nm, the Nd³⁺ excited to their metastable state (4F3/2) undergoes non-radiative energy transfer to Yb3+ ions, which subsequently emits luminescence at 980 nm. The back energy transfer (BET) from Yb3+ to Nd3+, mediated by phonon absorption, is thermally activated. As the temperature increases, the phonon density required for the BET process also increases, leading to an enhanced probability of BET. This, in turn, results in a reduced luminescence lifetime. This makes the NP emission strongly dependent on temperature, and it constitutes the working principle of our PL lifetime-based nanothermometers. Fitting the decay curves to a double exponential decay (\({I}_{{em}}\left(t\right)={A}_{1}\exp \left(-t/{\tau }_{1}\right)+{A}_{2}\exp \left(-t/{\tau }_{2}\right)\)) it is possible to define for each temperature an average fluorescence lifetime:
where \({A}_{1}\) and \({A}_{2}\) are the amplitudes of the decay components associated with lifetimes \({\tau }_{1}\) and \({\tau }_{2}\), respectively. The analysis of the decay curves obtained at different temperatures reveals a pseudo-linear decay of \(\bar{\tau }\) (Fig. 3c). The pseudo-linear reduction of \(\bar{\tau }\) with temperature makes it possible to remotely determine the stent temperature from the experimental detection of its fluorescence lifetime. Furthermore, the fluorescence lifetime is not affected by the presence of tissue, allowing for reliable sub-tissue thermal readings, as opposed to ratiometric measurements, which can lead to erroneous sub-tissue thermal readouts due to tissue-induced spectral distortions, and the consequent experimental variance values35,43. The robustness of fluorescence lifetime as a thermometric parameter for sub-tissue measurements has also been verified by repeating measurements in the presence of a 0.5 cm thick tissue. The fluorescence decay curves obtained in presence of tissue (Fig. 3d) show no significant differences to those obtained in the absence of tissue (Fig. 3b). Further evidence is shown in the \(\bar{\tau }\) vs \(T\) plot obtained in the presence of tissue (Fig. 3e), which results in a virtually identical curve as that obtained in the absence of tissue (Fig. 3c).
a Schematic drawing of the experimental procedure used to evaluate the lifetime-based sensing capability of our fluorescent stents. Luminescence decay curves of the fluorescent stent obtained under 800 nm optical excitation at different temperatures, in absence (b) and presence (d) of tissue, fluorescence was recorded at 980 nm, and the tissue thickness was set to 0.5 cm. The temperature dependence of the average lifetime obtained in the absence (c) and presence (e) of tissue, the data were derived from fitting the curves shown in (b) and (d) to Eq. (2) in the main text. Fluorescence decay measurements were each performed in triplicate (n = 3).
The analysis of the \(\bar{\tau }\) vs T curves leads to a lifetime-based relative thermal sensitivity of \({S}_{r}={\bar{\tau }}^{-1}d\bar{\tau }/{dT}\cdot 100 \% \approx {0.58 \% {^{\circ }}{\rm{C}}}^{{-}1}\) that is found to be comparable to the thermal sensitivities previously reported for other lifetime-based luminescent nanothermometers operating in the biological windows (See Supplementary Table S2 in Supplementary Information)35,44,45,46,47,48,49. This relative thermal sensitivity is in agreement with values published previously for Nd,Yb:NPs in different media31. Taking into account our experimental uncertainty in the determination of \(\bar{\tau }\) (\(\delta \bar{\tau }\approx 0.2\,{\rm{\mu s}}\), see Supplementary Fig. S5 and Supplementary Section S8 in Supplementary Information) we conclude that in our experimental conditions the temperature resolution (\(\delta T=\delta \bar{\tau }/\bar{\tau }\cdot {{S}_{r}}^{-1}\)) achievable with our fluorescent stent is close to 0.2 °C. This thermal resolution is, for instance, sufficient to reach a thermal diagnosis of an atheromatous plaque: temperature differences of up to 2 °C have been reported between different stages of plaque development25.
3D printed luminescent stent for remote thermal monitoring of intracoronary hyperthermia treatment
The sub-degree thermal resolution provided by our luminescent stent would enable accurate thermal monitoring during thermal treatment of plaques (requiring temperature increments above 10 °C) as well as the thermal treatment of thrombosis (requiring temperature increments in the 2–20 °C range)50,51,52. We explore the potential of our luminescent stent for intracoronary thermal monitoring by simulating a fiber-assisted photothermal treatment (Fig. 4a). A 1450 nm single-mode fiber laser was optically coupled to a catheter constituted by a single-mode fiber equipped with a deflecting mirror at its end (catheter used for intracoronary tomography). The catheter was inserted into the tube containing the luminescent stent and its tip was placed inside the stent (Fig. 4a). Then, the tube containing the stent was surrounded by a 0.5 cm thick tissue (Fig. 4a). Remote thermal reading was achieved by analyzing the fluorescence decay curve of Nd,Yb:NPs within the stent. Measurements were acquired five minutes after switching on the 1450 nm laser to ensure a stable temperature has been achieved (see Supplementary Fig. S6 and Supplementary Section S9 in Supplementary Information). The experimental set-up used for the collection of fluorescence decay curves was the same as in the experiments of Fig. 3. When the 1450 nm laser is off, the temperature of the stent is that of the water flowing through the tube (21 °C). When the 1450 nm laser is switched on, the 1450 nm laser radiation delivered at the end of the catheter is strongly absorbed by water leading to a local increment of temperature. Laser-induced internal heating is, indeed, evidenced by a slight (\(\Delta \bar{\tau }={-}2\,{\rm{\mu s}}\)) decrease in the average fluorescence lifetime of the stent (Fig. 4b). By using the calibration curve of Fig. 3c we can estimate local heating of the stent close to 2.1 °C. The experimental data can be compared with the numerical simulations of the internal temperature distributions performed as described in Supplementary Section S10 of Supplementary Information53,54,55,56. The simulated temperature profile along the vertical (y axis in Fig. 4c) shows a maximum temperature increment at the tip of the catheter close to 10 °C (Fig. 4e), with the temperature rapidly decreasing radially moving away from the catheter. The temperature variation at the location of the stent (horizontal “x” profile indicated in Fig. 4c) reveals a maximum temperature increment close to 1.5 °C (Fig. 4d), which is in reasonable agreement with the temperature increment determined experimentally (2.1 °C). Differences could arise from the fact that during the experiment, it is not possible to ensure that the tip of the catheter is located at the central position of the tube. Note that conventional methods used for thermal imaging in biomedical applications (such as infrared thermography) fail to provide the stent temperature as they only access the temperature at the tissue surface (see Supplementary Fig. S7 in Supplementary Information). Results included in Fig. 4 evidence the potential of luminescent stent for remote intracoronary thermal monitoring with a sub-degree resolution.
a Schematic drawing of the experimental procedure used to evaluate the potential of fluorescent stent for remote thermal monitoring of intracoronary hyperthermia treatment. Excitation and emission wavelengths were 800 nm and 980 nm, respectively. b Average fluorescence lifetime obtained in the absence and presence of 1450 nm radiation. The on-target 1450 nm laser power provided by the catheter was 20 mW. c Numerical simulations of the temperature distribution within the tube caused by the 1450 nm laser delivered by the catheter. d Temperature along the horizontal scan (x axis) indicated by the horizontal dashed line in (c). This scan (horizontal line) is parallel to the wall of the tube, and it is located at 0.2 mm to the wall of the tube (i.e. where the stent is placed). e Temperature along the vertical scan (y axis) indicated by the vertical dashed line in (c). In this profile, y = 0 corresponds to the location of the tip of the catheter.
Discussion
In summary, we report on the creation of a 3D printed multifunctional cardiovascular implant. In particular, the inclusion of neodymium and ytterbium co-doped nanoparticles during the printing process resulted in a luminescent stent prototype. The spectroscopic characterization of the 3D printed stent showed that the luminescent properties of neodymium and ytterbium co-doped nanoparticles remain intact during the fabrication process. The infrared luminescence generated by the lanthanide-doped nanoparticles allows the real-time location of the stent under physiologically relevant tissue thicknesses. In addition, we also demonstrate the ability of the 3D printed stent to provide remote and sub-tissue thermal information by lifetime-based luminescent thermometry. In turn, the high thermal sensitivity of our 3D printed stent enabled us to carry out proof-of-concept experiments to validate the implant as local monitor catheter-assisted photothermal treatment.
The synergy between the new capabilities in the field of additive manufacturing and the vast library of luminescent nanoparticles predicts the appearance of new coronary implants with previously unimagined functionalities and which could represent an unprecedented advance in intracoronary diagnosis and therapy.
Methods
Synthesis of Nd,Yb:NPs
NaYF4 inert cores were synthesized by a thermal decomposition method. Firstly, 1.5 mmol YCl3 was dissolved in 10 ml TFA in a flask at 90 °C overnight. Then TFA was evaporated under N2 atmosphere until the precursor Y(TFA)3 white power dried. Then the precursor powder was dissolved in a mixture of 3.2 ml OA, 3.2 ml OM, 6.4 ml ODE and 1.5 mmol NaTFA and heated to 110 °C for 30 min. After that, the temperature increased to 310 °C for 30 min under N2 atmosphere before cooling down to room temperature. Finally, the resultant cores were collected through centrifugation (8500 rpm for 10 min) after adding 5 mL ethanol, washed with ethanol three times, and finally dispersed in 10 mL hexane for further use.
NaYF4@NaYF4:Yb20,Nd60 core@shell nanoparticles were synthesized following a seed-mediated epitaxial growth procedure. Firstly, the precursor of Y(TFA)3, Yb(TFA)3, Nd(TFA)3 was synthesized using the same procedure as for the precursor Y(TFA)3, except that 0.2 mmol of YCl3·6H2O, 0.2 mmol of YbCl3·6H2O, and 0.6 mmol of NdCl3·6H2O were used. Then the precursor powder was mixed with a mixture of 10 ml OA, 10 ml ODE and 1.0 mmol NaTFA together with 0.5 mmol of the previously synthesized core and heated to 120 °C for 30 min. After that, the temperature increased up to 300 °C for 30 min under N2 atmosphere before cooling down to room temperature. Finally, the resultant cores were collected through centrifugation (8500 rpm for 10 min) after adding 5 mL ethanol, washed with ethanol three times, and finally dispersed in 10 mL hexane for further use.
NaYF4@NaYF4:Yb20,Nd60@CaF2 core@shell@shell nanoparticles were synthesized following a hot injection method. Firstly, the Ca(TFA)2 precursor was prepared using the same procedure as precursor Y(TFA)3 except that 1 mmol of CaCl2 were used. Then the mixture of CaF2 precursor and 3 ml OA in flask were heated to 150 °C for 30 min before cooling down to room temperature for further use. Briefly, the NaYF4@NaYF4:Yb20,Nd60 core@shell nanoparticle was used as cores for a seed-mediated growth of a CaF2 shell. Then a mixture of NaYF4@NaYF4:Yb20,Nd60 core@shell NPs (5 mL, ~20 mg/mL), 7 mL OA, and 7 mL ODE were heated to 110 °C for 30 min under N2 atmosphere and then increased to 310 °C. Subsequently, 0.5 mmol of Ca(TFA)2 dissolved in 1.5 mL OA was injected into the solution divided in 10 different injections (0.15 mL each) at intervals of 10 min. The resultant core@shell@shell RENPs were precipitated, washed with ethanol, and finally dispersed in 10 mL hexane.
Fabrication of 3D printed luminescent stent
Firstly, the NaYF4@NaYF4:Yb20,Nd60@CaF2 nanoparticles were concentrated by rotary evaporator (34 mg/ml). The mixture of selected acrylic resin (10 g, Elegoo) with concentrated nanoparticles (250 μl) were sonicated and stirred approximately 10 min before pouring into the resin tank of the LCD 3D printer (Creality Halot One). The lateral pixel size is 50 μm, and the printer platform and cuvette had been modified to work with small volumes. The model is cured by UV light in a layer-by-layer fashion, where the layer thickness is set at 50 μm, and the exposure time at 3.1 s per layer. Several stents are printed simultaneously, and once the print is completed, they are removed from the printer and rinsed with isopropanol.
Mechanical properties of 3D printed luminescent stent
Uniaxial compression tests were carried out on an Instron 3400 Series Universal Testing Machine with a 500 N load cell. Samples were compressed until failure at a rate of 0.1 mm/min, with a preload of 0.05 N. For these measurements, the stents were compressed in the radial direction, instead of the usual axial direction, as this more closely resembles the stress that the stents will undergo once implanted. We evaluated stiffness, rather than Young modulus, motivated by the fact that stiffness is an extensive property of our stents, while Young modulus is an intensive property of the material they are made of (acrylic resin), independent of size and shape.
Fluorescence emission spectroscopy of 3D printed luminescent stent
The emission spectra were collected under 980 nm continuous wavelength laser irradiation and 850 nm long-pass filter (FEL850 Thorlabs) was used to remove the laser contribution. The luminescence was acquired with a NIR Quest spectrometer (900–2500 nm wavelength) from Ocean Insight.
Fluorescence lifetime and the relative thermal sensitivity of 3D printed luminescent stent
The photoluminescence decay curves of in-tube stents were obtained by excitation of the stent through chicken breast tissue with an OPO oscillator (Quanta Ray) adjust to 800 nm that provides 8 ns pulses at a repetition rate of 10 Hz. The PL intensity decay curve was detected with a Peltier-cooled photomultiplier tube with enhanced sensitivity in the NIR (Hamamatsu R5509-73). The contribution of scattered laser radiation was removed by using long pass filter (FEL850 from Thorlabs) and a monochromator (Shamrock 320 from Andor). The time evolution of the PL signal was finally obtained and averaged by a digital oscilloscope (LeCroy WaveRunner 6000). The temperature of the stent was changed by the chiller that connected to the tube. And the relative thermal sensitivity \({s}_{r}\) was defined as Eq. (3) from the fitting of photoluminescence decay curves.
where τ is average fluorescence lifetime and \(T\) is the temperature.
In vitro cytotoxicity measurement
The 3D printed luminescent stent was tested for safety in vitro using mouse aortic endothelial cells (primary culture, reference: P10427) that provided by Innoprot are isolated from Swiss mouse aorta from Innoprot (Innoprot, Bizkaia, Spain) and then analyzed using the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazoliumbromide) colorimetric assay (Sigma Aldrich, St Louis, USA).
Cells were trypsinized, counted using a Neubauer chamber and seeded into 96-well culture plates at a density of 2000 cells/well before being incubated overnight in a CO2 incubator with 200 µL complete culture medium (Endothelial cell medium, Innoprot).
At the same time, the stent that was ground into powder dispersed in PBS buffer and diluted stepwise into the following four concentrations, 300 μg/ml, 150 μg/ml, 75 μg/ml, 37.25 μg/ml. These concentrations were added to the wells in quintuplicate. After additional 24 h incubation, cells were washed with PBS twice after removing the culture medium, 180 μl culture medium and 20 μl of MTT was added and incubated for 1.5–2 h in darkness, at 37 °C. Once the formazan crystals were evident, the reaction was stopped by carefully aspirating the MTT solution, and crystals were then dissolved by adding 100 μL of DMSO (Sigma Aldrich). Afterwards, wells were incubated in darkness at 37 °C and gently shaked for 15 min. Finally, absorbance was measured in a spectrum 555 nm in an Epoch™ 2 Microplate Spectrophotometer (Biotek) in duplicates.
Penetration depths
Penetration depths were measured with chicken breast tissue that was freshly purchased in supermarket, and multiple slices with varying thicknesses ranging from 0.66 cm to 4.09 cm were cut. They were stored in plastic foil whenever they were not measured. During measurements, the tissue was kept at room temperature.
The 3D printed luminescent stent within the tubing was placed on a platform after covering with chicken breast tissue at different thickness (thickness: 0.00 cm, 0.66 cm, 1.04 cm, 1.50 cm, 1.82 cm, 2.37 cm, 3.06 cm, 4.09 cm). The tissue and stent were irradiated by 800 nm continuous wavelength laser beams (140 mW/cm2) from above, the fluorescence images collected by a InGaAs camera that contains an 850 nm long pass filter while under laser irradiation. Finally, the fluorescence intensity was analyzed by ImageJ. The effective penetration depth was obtained by linear fitting of the logarithm of fluorescence intensity to the different tissue thickness, following Eq. (1) from the text.
Data availability
The data that supports the findings of this study are available from the corresponding author upon reasonable request.
References
Scafa Udriște, A., Niculescu, A.-G., Grumezescu, A. M. & Bădilă, E. Cardiovascular stents: a review of past, current, and emerging devices. Materials 14, 2498 (2021).
Kapoor, A. et al. The road to the ideal stent: a review of stent design optimisation methods, findings, and opportunities. Mater. Des. 237, 112556 (2024).
Herbert, R., Lim, H.-R., Rigo, B. & Yeo, W.-H. Fully implantable wireless batteryless vascular electronics with printed soft sensors for multiplex sensing of hemodynamics. Sci. Adv. 8, eabm1175 (2022).
Herbert, R. et al. Fully implantable batteryless soft platforms with printed nanomaterial-based arterial stiffness sensors for wireless continuous monitoring of restenosis in real time. Nano Today 46, 101557 (2022).
Fu, D., Yang, F., Zhang, J., Xiang, Z. & Wang, Y. Near-infrared rechargeable persistent luminescence nanoparticles for biomedical implants in vivo noninvasive bioimaging. ACS Appl. Mater. Interfaces 15, 53310–53317 (2023).
Pei, P. et al. NIR-II ratiometric lanthanide-dye hybrid nanoprobes doped bioscaffolds for in situ bone repair monitoring. Nano Lett. 22, 783–791 (2022).
Liu, J. et al. Long-term in vivo glucose monitoring by polymer-dot transducer in an injectable hydrogel implant. Anal. Chem. 94, 2195–2203 (2022).
Chu, C. et al. A near-infrared light-triggered shape-memory polymer for long-time fluorescence imaging in deep tissues. J. Mater. Chem. B 8, 8061–8070 (2020).
Deng, Z. et al. A general strategy for designing NIR-II emissive silk for the in vivo monitoring of an implanted stent model beyond 1500 nm. J. Mater. Chem. B 8, 4587–4592 (2020).
Fan, W. et al. Enhanced afterglow performance of persistent luminescence implants for efficient repeatable photodynamic therapy. ACS Nano 11, 5864–5872 (2017).
Kim, S. H. et al. NIR fluorescence for monitoring in vivo scaffold degradation along with stem cell tracking in bone tissue engineering. Biomaterials 258, 120267 (2020).
Jing, L. et al. Noninvasive in vivo imaging and monitoring of 3D-printed polycaprolactone scaffolds labeled with an NIR region II fluorescent dye. ACS Appl. Bio Mater. 4, 3189–3202 (2021).
Lee, M. A. et al. In-vivo fluorescent nanosensor implants based on hydrogel-encapsulation: investigating the inflammation and the foreign-body response. J. Nanobiotechnology 21, 133 (2023).
Esfahani, G., Lucas, H., Syrowatka, F. & Mäder, K. A starch-based implant as a controlled drug release system: non-invasive in vivo characterization using multispectral fluorescence imaging. J. Controlled Release 358, 358–367 (2023).
Iglesias-Mejuto, A. et al. 3D-printed aerogels as theranostic implants monitored by fluorescence bioimaging. Bioact. Mater. 41, 471–484 (2024).
Yue, L. et al. Rechargeable afterglow superclusters for NIR-excitable repetitive phototherapy. Nano Lett. 24, 15406–15413 (2024).
Wang, X. et al. In situ imaging and anti-inflammation of 3D printed scaffolds enabled by AIEgen. ACS Appl. Mater. Interfaces 15, 25382–25392 (2023).
Qiao, R. et al. Molecular imaging of vulnerable atherosclerotic plaques in vivo with osteopontin-specific upconversion nanoprobes. ACS Nano 11, 1816–1825 (2017).
Diao, S. et al. Biological imaging without autofluorescence in the second near-infrared region. Nano Res. 8, 3027–3034 (2015).
Smith, A. M., Mancini, M. C. & Nie, S. Second window for in vivo imaging. Nat. Nanotechnol. 4, 710–711 (2009).
Hemmer, E., Benayas, A., Légaré, F. & Vetrone, F. Exploiting the biological windows: current perspectives on fluorescent bioprobes emitting above 1000 nm. Nanoscale Horiz. 1, 168–184 (2016).
Lee, S. Y., Lin, M., Lee, A. & Park, Y. I. Lanthanide-doped nanoparticles for diagnostic sensing. Nanomaterials 7, 411 (2017).
Ximendes, E. C., Rocha, U., Kumar, K. U., Jacinto, C. & Jaque, D. LaF3 core/shell nanoparticles for subcutaneous heating and thermal sensing in the second biological-window. Appl. Phys. Lett. 108, 253103 (2016).
Ximendes, E. C. et al. In vivo subcutaneous thermal video recording by supersensitive infrared nanothermometers. Adv. Funct. Mater. 27, 1702249 (2017).
Madjid, M. et al. Thermal detection of vulnerable plaque. Am. J. Cardiol. 90, L36–L39 (2002).
Ley, O. & Kim, T. Determination of atherosclerotic plaque temperature in large arteries. Int. J. Therm. Sci. 47, 147–156 (2008).
Lasanen, R. et al. Thermal imaging in screening of joint inflammation and rheumatoid arthritis in children. Physiol. Meas. 36, 273 (2015).
Shen, Y. et al. Reliable and remote monitoring of absolute temperature during liver inflammation via luminescence-lifetime-based nanothermometry. Adv. Mater. 34, 2107764 (2022).
Stefanadis, C. et al. Thermal heterogeneity within human atherosclerotic coronary arteries detected in vivo: a new method of detection by application of a special thermography catheter. Circulation 99, 1965–1971 (1999).
Refaat, A. et al. Activated platelet-targeted IR780 immunoliposomes for photothermal thrombolysis. Adv. Funct. Mater. 33, 2209019 (2023).
Verheye, S., De Meyer, G. R. Y., Van Langenhove, G., Knaapen, M. W. M. & Kockx, M. M. In vivo temperature heterogeneity of atherosclerotic plaques is determined by plaque composition. Circulation 105, 1596–1601 (2002).
Hamraoui, K. et al. Exploring the origin of the thermal sensitivity of near-infrared-II emitting rare earth nanoparticles. ACS Appl. Mater. Interfaces 15, 32667–32677 (2023).
Li, D., Jia, M., Jia, T. & Chen, G. Ultrasensitive NIR-II ratiometric nanothermometers for 3D in vivo thermal imaging. Adv. Mater. 36, 2309452 (2024).
Jaque, D. et al. Nd3+→Yb3+ energy transfer in the YAl3(BO3)4 nonlinear laser crystal. Phys. Rev. B 68, 035118 (2003).
Tan, M. et al. Accurate in vivo nanothermometry through NIR-II lanthanide luminescence lifetime. Small 16, 2004118 (2020).
Wei, Y., Wang, M., Zhao, D., Li, H. & Jin, Y. Structural design of mechanical property for biodegradable polymeric stent. Adv. Mater. Sci. Eng. 2019, 2960435 (2019).
Sordillo, L. A., Pu, Y., Pratavieira, S., Budansky, Y. & Alfano, R. R. Deep optical imaging of tissue using the second and third near-infrared spectral windows. J. Biomed. Opt. 19, 056004 (2014).
Feng, Z. et al. Perfecting and extending the near-infrared imaging window. Light Sci. Appl. 10, 197 (2021).
Xiong, L., Yang, T., Yang, Y., Xu, C. & Li, F. Long-term in vivo biodistribution imaging and toxicity of polyacrylic acid-coated upconversion nanophosphors. Biomaterials 31, 7078–7085 (2010).
ANSI Z136.3 - safe use of lasers in health care. Laser institute of America 13501 Ingenuity Drive, Suite 128 Orlando, FL 32826 (2018)
Welsher, K., Sherlock, S. P. & Dai, H. Deep-tissue anatomical imaging of mice using carbon nanotube fluorophores in the second near-infrared window. Proc. Natl. Acad. Sci. 108, 8943–8948 (2011).
Foin, N. et al. Incomplete stent apposition causes high shear flow disturbances and delay in neointimal coverage as a function of strut to wall detachment distance. Circ. Cardiovasc. Interv. 7, 180–189 (2014).
Marciniak, L. & Trejgis, K. Luminescence lifetime thermometry with Mn3+ –Mn3+ co-doped nanocrystals. J. Mater. Chem. C 6, 7092–7100 (2018).
Balabhadra, S., Solanki, P. S., Reid, M. F. & Wells, J.-P. R. Lifetime thermometry in the third biological window using KMnF3:Yb/Er nanoparticles. Opt. Laser Technol. 171, 110438 (2024).
Kong, M. et al. Luminescence interference-free lifetime nanothermometry pinpoints in vivo temperature. Sci. China Chem. 64, 974–984 (2021).
Raab, M. E., Maurizio, S. L., Capobianco, J. A. & Prasad, P. N. Lifetime of the3 H4 electronic state in Tm3+-doped upconverting nanoparticles for NIR nanothermometry. J. Phys. Chem. B 125, 13132–13136 (2021).
Sun, Y. et al. Nanothermometer for in vivo temperature detection with high spatial resolution based on core–shell rare earth nanoparticles. ACS Appl. Nano Mater. 6, 23173–23183 (2023).
Wu, L., Jia, M., Li, D. & Chen, G. Shell engineering on thermal sensitivity of lifetime-based NIR nanothermometers for accurate temperature measurement in murine internal liver organ. Nano Lett. 23, 2862–2869 (2023).
Zhang, M., Wang, B., Cai, Y., Jin, D. & Zhou, J. Thermally prolonged NIR-II luminescence lifetimes by cross-relaxation. Nano Lett. 24, 4877–4884 (2024).
Ellenbroek, G. H. J. M. et al. Radiofrequency ablation of the atherosclerotic plaque: a proof of concept study in an atherosclerotic model. J Cardiovasc. Transl. Res. 10, 221–232 (2017).
Zhao, S. et al. A new radiofrequency balloon angioplasty device for atherosclerosis treatment. Biomed. Eng. OnLine 19, 44 (2020).
Singh, N., Varma, A., Verma, A., Maurya, B. N. & Dash, D. Relief from vascular occlusion using photothermal ablation of thrombus with a multimodal perspective. Nano Res. 9, 2327–2337 (2016).
Bergman, T. L. Fundamentals of heat and mass transfer (John Wiley & Sons, 2011).
Douglas, J. & Gunn, J. E. A general formulation of alternating direction methods. Numer. Math. 6, 428–453 (1964).
Lifante, J. et al. Early in vivo detection of denervation-induced atrophy by luminescence transient nanothermometry. J. Biophotonics 17, e202300249 (2024).
Ramires, M. L. V. et al. Standard reference data for the thermal conductivity of water. J. Phys. Chem. Ref. Data 24, 1377–1381 (1995).
Acknowledgements
This work was supported by Grant PID2023-146775OB-I00 (INCLINA) and Grant PID2021-123318OB-I00(NANOGRANZ) funded by MCIN/AEI/ 10.13039/501100011033 and the European Regional Development Fund (ERDF). Dongmei Qiu acknowledges the scholarship from China Scholarship Council (No.202308330053). Juan Pedro Cascales acknowledges support from the Comunidad de Madrid, Spain, through an “Atracción de Talento Investigador” fellowship (2022-T1/BMD-23751). Jose Marques-Hueso thanks the Spanish Ministerio de Universidades for support through the program Beatriz Galindo (Award BG22/00128).
Author information
Authors and Affiliations
Contributions
Daniel Jaque and Jorge Rubio Retama designed the study. Dongmei Qiu, Paula Gutiérrez González, M Carmen Iglesias de la Cruz, Pablo Molina, Juan Pedro Cascales, Gines Lifante Pedrola, Jose Marques-Hueso, Sergio Royuela, Daniel Jaque and Jorge Rubio Retama collected the data. Dongmei Qiu, Daniel Jaque and Jorge Rubio Retama performed statistical analysis and wrote the initial draft. Paula Gutiérrez González, M Carmen Iglesias de la Cruz, Pablo Molina, Juan Pedro Cascales, Gines Lifante Pedrola, Sergio Royuela and Jose Marques-Hueso reviewed and edited the manuscript. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qiu, D., Gutiérrez González, P., Iglesias de la Cruz, M.C. et al. Infrared emitting lanthanide doped nanoparticles provide sensing capabilities to coronary implants. npj Nanophoton. 2, 18 (2025). https://doi.org/10.1038/s44310-025-00067-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44310-025-00067-4