Abstract
Both parasite manipulation of host behavior and the roles of circadian clocks in infectious disease are not well understood. However, studies into parasite-manipulated insects suggest that host rhythms are altered at different levels of biological organization. Here, we discuss this hypothesis in the context of circadian plasticity. We argue that striking overlap between manipulation mechanisms and plastic functioning of the insect clock exists across independently evolved parasite-host systems. As such, investigating parasitic behavioral manipulation provides an opportunity to better understand circadian plasticity and how infection and clocks intersect across taxa.
Similar content being viewed by others
Introduction
Environmental factors including light, temperature, food availability, social dynamics and predation threat fluctuate across daily, monthly, seasonal and annual cycles. To anticipate these cycles, organisms throughout nature have evolved rhythmic physiology and behaviors tailored to their unique ecological niches and challenges1,2. This includes (endo)parasites: organisms that invade and take advantage of the resources of another organism - their host. Parasites need to coordinate their activities to external abiotic fluctuations and fluctuations within the host. For instance, daily rhythms in host immune responses have provided selection pressures for parasites to time host invasion and circumvent phases of high immunity, thereby increasing infection success3. Exploring the roles of biological clocks in parasite-host interactions and co-evolution could, therefore, contribute to our understanding of these ecologically ubiquitous interactions and improve treatment for clinically and agriculturally relevant infections.
Organismic rhythms are driven by endogenous, robust molecular clocks. These clocks consist of negative transcription-translation feedback loops, that entrain to cycling environmental factors (i.e., Zeitgebers) and regulate oscillations in gene expression and protein production4 (Fig. 1). Since circadian clocks and their outputs exist to anticipate predictable 24 h rhythms, they should also be somewhat plastic to allow for responses to both gradual and acute changes in the environment (Fig. 1). This plasticity can be observed when matters such as food availability and social context are involved. For instance, mosquitoes, exhibit periodicity in their flight and blood-feeding activity5, which are modulated by temperature and the mosquito’s nutritional status6,7,8. Similarly, nocturnal mice gradually adjust their active phase when food is experimentally restricted to the daytime, being able to quickly reverse again when these restrictions are lifted9,10. Moreover, the honeybee Apis mellifera, uses social cues related to food availability to synchronize circadian foraging times among colony members11. Flexible rhythmicity is also observed in organisms as they age. When aging, adult humans typically transition to an earlier chronotype12 while worker ants and bees shift from seemingly aperiodic activity to 24 h rhythms to fulfil respective nurse and forager caste roles in the colony13. However, when sudden disruptions in colony organization or changes in social context occur, foragers can also quickly become nurses again, and vice versa14. Additionally, endoparasites, such as microfilaria and malaria parasites, show adaptive flexibility in their transmission timing to align to the daily activity peaks of their specific mosquito vectors15,16,17.
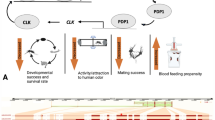
A visual summary of the hypothesis that parasites manipulate host behavior by targeting their circadian plasticity, explained at three levels of biological organization: molecular, cellular and behavioral phenotypes. a The molecular clock consists of a handful of interacting core circadian genes that create robust transcription-translation feedback loops (yellow). These interactions repeat in a ~24 h rhythm and entrain many downstream genes to cycle in their expression levels (blue). The dotted circle symbolizes the surrounding environment of an organism, which contains multiple factors that can act as predictable 24 h Zeitgebers and present disturbances that require some level of clock plasticity. b Cellular and molecular mechanisms of circadian plasticity. Red boxes include examples of parasitic manipulation on both levels. Cell bodies of clock neurons (magenta) and glial cells (teal) are illustrated in an ant brain slice (adapted from90). c Examples of rhythmic output that can be measured at the behavioral level in insects (e.g. ant, caterpillar and fly) and how these rhythms may change because of environmental factors or parasitic manipulation.
While circadian plasticity provides obvious fitness benefits, it could also keep the door open for unwanted modulation and corruption. Parasites cannot only adapt timed strategies to match host activity and circumvent heightened immune response, but in cases of prolonged co-evolution also manipulate and weaken this response18. Some parasites even enhance the intimate relationship with their host by adaptively manipulating their morphological or behavioral phenotypes to benefit parasite fitness. These extended behavioral phenotypes often involve abnormal daily timing, begging the question whether circadian clocks play a prominent role in parasite manipulation of host behavior. While the molecular mechanisms that underlie this phenomenon are currently not clear, hijacking an existing, somewhat flexible host system that modulates multiple behaviors provides a potential opportunity for enhancing parasite fitness. Here, we make a case for the host circadian system to be a putative, convergently evolved target for manipulating parasites, because a) it regulates many downstream behaviors and b) it is plastic. We do so by discussing the topics of circadian plasticity and parasitic behavioral manipulation at different levels of biological organization: 1) organismic, 2) cellular, and 3) molecular (Fig. 1). We hereby mostly focus on insects and their manipulating parasites since these interactions have currently been studied most extensively in the context of the circadian clock.
Altered circadian timing: a convergently evolved parasite extended phenotype
To optimize their fitness, some parasites act through the host body to influence rhythmic activity patterns (Fig. 1). Prime examples involve fungi that invade and manipulate insect hosts, including the behavioral changes observed in ‘zombie’ ants infected by Ophiocordyceps. Infected ants lose their otherwise circadian foraging activity, become hyperactive and walk around seemingly directionless throughout the light/dark cycle. This is coupled with a loss of communication and collaboration with nest mates19,20. These behavioral changes are thought to promote fungal fitness by minimizing host interactions with nest mates that attack infected ants as a social immunity response threatening completion of the fungal life cycle21.
After two to three weeks of infection, the ant shifts from directionless arrhythmic hyperactivity to targeted, timed climbing of vegetation close to its end of life20,22, referred to as summiting. In nature, summiting is concluded around solar noon when the ant attaches itself at an elevated position by biting into the vegetation with its mandibles23. Such timed summiting is also observed across other fungus-infected insects, including flies infected by Entomophthorales24. These fungi reside in a different phylum from Ophiocordyceps and induce summit behaviors around dusk. Though summiting is synchronized to different times of day for Ophiocordyceps and Entomopthorales fungi, the adaptive function appears to be similar: placing the insect host under environmental conditions that favor spore production and eventually, transmission. Increased humidity from dusk till dawn promotes the quick sprouting of infectious Entomophthora conidia from fresh fly cadavers25. Instead, for Ophiocordyceps the development of spores and their dispersal can take up to several months26. To assure an optimal microclimate that will both preserve the cadaver during that time and promote eventual fruiting body (spore-bearing structure) formation, Ophiocordyceps-infected ants attach themselves at very specific incident light levels under the canopy26. When light conditions in the field were experimentally altered, the production of Ophiocordyceps fruiting bodies was reduced27 and moving the cadaver itself completely halted development28. Summiting and subsequent death of infected ants occurring when sun intensity peaks might, therefore, promote fitness by facilitating the seeking of ideal conditions for spore formation. Following fungal spore dispersal is also timed, seemingly to coincide with the host’s peak activity to maximize transmission opportunities. Ophiocordyceps releases spores around dusk to infect nocturnal forager ants while Entomophthora muscae targets diurnal flies at dawn29,30. Taken together, these manipulating fungal insect parasites appear to have evolved to both anticipate the daily activity rhythms of healthy hosts and induce altered rhythms upon infection. Being able to modulate and anticipate host timing are likely parasite-adaptive traits since failure to produce infective propagules or spreading them at the wrong time would jeopardize transmission success and parasite survival.
Affecting host timing appears to play an adaptive role in non-fungal parasites as well. Baculoviruses that infect caterpillars show parallels with fungal extended phenotypes as they also increase locomotion activity and induce climbing behaviors in moth species such as Lymantria dispar31. The climbing behavior of these moth larvae is restricted to the nighttime to facilitate feeding, while inactivity during the day promotes survival by avoiding predation, parasitoids and overheating32,33. Baculovirus infection disrupts this daily climbing rhythm - larvae remain in their elevated positions where they eventually die and liquify. This elevation promotes transmission of infectious particles either by them raining down across a larger area of vegetation, or through transportation by birds that can spot the infected caterpillars more easily31,34,35,36. While this might suggest that timed extended phenotypes only occur in strictly terrestrial parasite-host systems that involve summiting, the life cycle of horsehair worms also involves timed host manipulation. These parasites induce terrestrial hosts such as crickets37 and mantids38 to jump into bodies of water. During the nighttime, infected hosts become attracted to reflective light coming from water puddles39. By sensing horizontally polarized light to distinguish water depth, hairworm-infected mantids specifically steer towards deeper, perennial ponds to increase the parasite’s mating chances40.
Though convergence in behavioral manipulation phenotypes exists across natural parasite-host systems, variation in these systems constitutes a challenge in understanding behavioral manipulation at an organismal level. Additionally, observations are inherently biased by the human perspective. It is difficult to imagine what senses beyond our own play determining roles in other species. As such, we likely overlook many extended behaviors relevant for specific ecological parasite niches. The challenge, therefore, lies in taking the host perspective to understand how its parasite found ways to manipulate it to its advantage41. Moreover, descriptions of extended phenotypes across species evoke questions about their underlying molecular causal mechanisms and whether those have convergently evolved. To explore this, the following two sections will outline our current knowledge on how plastic and manipulated circadian rhythms are regulated in the brain on 1) a cellular and 2) a molecular level (Fig. 1).
Heterogeneous cellular networks facilitate plasticity of the circadian clock and rhythmic behavior
The basis of (adaptable) behavioral circadian rhythms lies in diverse and interconnected brain cells that keep time by expression of the molecular clock, entrain downstream cells and ultimately regulate cyclic behavior. The most extensive research into clock cellular network organization and how it relates to circadian plasticity has been conducted in Drosophila. The fly clock cellular network consists of 120 cells per hemisphere (only around 0.1% of a fly brain)42, and is divided into 15 lateral and 105 dorsal clock neurons (LNs and DNs). The smaller population of LNs has been the focal point of insect circadian research in the past since these cells generate endogenous rhythmicity regardless of external cues43. Additionally, small ventral LNs (sLNv) are easier to study than DNs, because they are fewer in number, anatomically distinct and easily targeted with functional genetic tools42,44. Only recently the heterogeneity within DNs has been characterized in terms of connectivity, morphology and gene expression based on connectome42,44 and single-cell transcriptomic work45,46. These high-resolution datasets open new possibilities to explore the functional roles of diverse DNs in regulating downstream rhythmic behavior. As they depend on external Zeitgebers, DNs modulate, rather than generate behavioral rhythms according to those environmental cues (as reviewed in refs. 47,48,49). Therefore, DNs appear most relevant for dynamically regulating the adaptation of rhythmic behavior in natural settings (Fig. 1).
The clock cell network primarily relies on paracrine signaling via neuropeptides to communicate to downstream targets and regulate animal behavior. Neuropeptides act slower but last longer than synaptic neurotransmitters, enabling dynamic adaptations to environmental conditions50. Examples include pigment-dispersing factor (PDF) and allatostatin, which is known to inhibit secretion of juvenile hormone (JH), an important insect hormone implicated in development and behavior across insect species51,52,53, including ant behavior manipulation20 and behavioral caste division54,55. The composition of these neuropeptides differs significantly between cell subtypes, contributing to functional heterogeneity within the circadian system42,56. Furthermore, different neuropeptides appear to regulate circadian behavior in flies as compared to mammals and even other insect species. This suggests that the mode of signaling – paracrine – may be more critical than the specific molecule itself57. One class of DN downstream targets are the ~80 neurosecretory cells42,56, which regulate many plastic circadian behaviors such as feeding. One heterogeneous subset of these neurosecretory cells is located in the pars intercerebralis (PI) region - the fly functional homolog of the mammalian hypothalamus. The PI acts as a relay between circadian DNs and two endocrine glands located around the aorta and gut56. Importantly, the neuropeptidergic and hormonal signaling pathways within the circadian clock network appear to be key outputs to the endocrine system. This puts the clock in control of a wide array of behaviors and physiology42,56 and would make it an effective pathway for parasitic manipulators to hijack.
Besides dorsal clock neurons, non-neuronal, abundant and diverse glial cells may contribute to circadian plasticity (Fig. 1). Glia respond to metabolic and immune stressors such as temperature changes or infections in a circadian-dependent manner58,59. Unlike other non-clock neurons, they do express circadian clock genes60 and have the highest number of cycling genes compared to other cell types61, highlighting their important role in regulating circadian processes. In flies, the blood-brain barrier – more accurately a hemolymph barrier formed by glia - increases permeability at nighttime62. This circadian plasticity of the barrier allows for the timed flux of hormones, affecting the sleep:wake cycle by stimulating lipid metabolism in a specific glial subtype63. Furthermore, plastic remodeling of circadian neurons is enabled by sphingolipid metabolism in glia64. These studies indicate that glial cells, like DN clock neurons, may sense environmental changes and flexibly shape circadian-driven output via hormonal and paracrine signaling. This makes the clock system plastic, but also vulnerable to parasitic manipulation.
Parasite strategies to manipulate host circadian cellular networks
While much remains unknown about the various cells within the clock network and how they orchestrate downstream circadian behaviors, even less is understood about interactions between the circadian clock networks of two different organisms. This axis of integration likely occurs to some extent in every endoparasitic interaction in which parasite and host have their own molecular clock. However, it seems most pronounced in interactions where host circadian outputs are measurably altered (e.g., malaria65 and sleeping sickness66) or even manipulated. Such rhythmic disease phenotypes provide an opportunity to study how clock networks intersect with infection and give rise to altered (behavioral) outputs.
To achieve precise behavioral manipulation, parasites must employ strategies to infiltrate and affect brain cells while maintaining their functionality. This may occur either by physical invasion of brain tissue or by altering brain network activity through effector release. Evidence for the former has been described in E. muscae-infected Drosophila. Entomophthora was shown to occupy space around the axons of posterior dorsal clock neuron type 1 (DN1p) and the dendrites of neurosecretory PI neurons67. A subset of these dendrites connects to an endocrine gland, the corpora allata (CA), which synthesizes and releases JH. Given the known roles of these players, it was hypothesized that Entomophthora hijacks the function of DN1p clock neurons and downstream PI-CA connectivity. Indeed, silencing or activating subsets of DN1p or PI neurons in Drosophila affected summiting67. Knocking down diuretic hormone 31(DH31) and CNMamide (CNMa) in the same DN1p subsets also reduced summiting, indicating that DN1p uses these peptides for signaling. However, summiting was not significantly impaired in fly mutants for DH31 and CNMa receptors. While this raises questions about the signaling mechanism, this discrepancy may be due to the unspecific fly-wide receptor disruption employed in these studies. Notably, disrupting sLNv neurons did not elicit the summiting phenotype, suggesting that the endogenous clock is not targeted by the fungus67. These findings are consistent with the notion that DNs, and not sLNv cells, sense the environment and modulate downstream circadian processes. Moreover, this study found that the fly glial hemolymph barrier was more permeabilized in Entomophthora-infected flies, possibly to enable easy passage of fungal behavior-manipulating effector proteins. While the increased permeability could be due to general barrier destruction, it may also be explained by the parasite exploiting the circadian-dependent flexibility provided by clock neurons and glia. These hypotheses could be tested by increasing the temporal resolution in which infected flies are studied to characterize the timeline of circadian system disruption.
Contrary to Entomophthora invasion into infected fly brains, both light and electron microscopy of Ophiocordyceps-infected ants indicate that these fungi do not penetrate brain tissue but invade muscle instead23,68,69. Perhaps other cells, that regulate circadian rhythms more generally, are targeted to disrupt and change circadian processes. Alternatively, Ophiocordyceps could be secreting effector peptides, proteins and metabolites to interact with host clock or clock-controlled proteins. Recent multi-omics work on Ophiocordyceps-infected ants suggests that this might be the case. However, this exploratory work was done on whole ant heads without taking brain heterogeneity into account. More detailed cellular level studies to investigate the brains of parasite-manipulated insects that exhibit circadian phenotypes and in which the parasite does not actively invade the brain tissue, would fill this knowledge gap. Such work would not only connect organismal level extended phenotypes with molecular underpinnings but also shine a light on the regulation of circadian plasticity and how it gives rise to rhythmic behaviors in general.
Molecular mechanisms of circadian clock plasticity
To better understand the molecular mechanisms underlying circadian plasticity and behavior manipulation, one can interrogate the genetic basis of organisms evolving and exploiting circadian plasticity to either adapt to changing environments or manipulate behavior of another organism. While canonical clock genes oscillate in synchrony between clock cell types, calcium activity peaks differently across those groups, which is facilitated by neuropeptide modulation. This asynchrony was hypothesized to underlie the generation of diverse downstream behavioral rhythmic outputs70. Furthermore, the phases and regulatory program of circadian genes vary between clock neurons and glia61,71,72 (Fig. 1). For example, the accessibility of timeless (tim) cis-regulatory regions differs between clock neurons and glial cells in Drosophila72. External cues also modulate the molecular clock. To adapt to extreme light conditions in high-latitude environments, Drosophila flies evolved a novel tim allele, ls-tim encoding both a longer and shorter TIM isoform (L-TIM and S-TIM)73. LS-TIM is less sensitive to light due to a weaker interaction with the circadian photoreceptor CRYPTOCHROME74. This reduced light sensitivity allows temperature cues to dominate circadian entrainment over light synchronization, thereby increasing fitness in environments with long photoperiods in the summer75. Furthermore, the cis-regulatory regions of neuropeptide-encoding gene Pdf, which regulates circadian locomotor rhythms, are different in globalist Drosophila melanogaster compared to Drosophila sechellia76. The latter species is endemic to the equatorial Seychelles Islands characterized by ~12:12 light:dark conditions throughout the year. Specifically, in D. melanogaster pdf RNA abundance dynamically adapts to different Light-Dark (LD) regimes and is overall higher compared to D. sechellia76. These RNA levels correlate with a green fluorescent reporter expression that was lower and more constant when driven by the pdf regulatory region of D. sechellia compared to the one of D. melanogaster. Moreover, reproductive success was reduced in D. sechellia, but not D. melanogaster, when exposed to an LD cycle with a long photoperiod. This further highlights the evolutionary pressure that likely molded differential behavioral plasticity between these species and suggests that affecting the interaction capacity of gene regulatory regions is a putative mechanism through which behavior could be manipulated.
Examples of natural timing variation, regulated by the molecular circadian clock, have also been described beyond flies. The behavior of the marine midge Clunio marinus, which is under circadian and circalunar control, shows different adaptations across strains. This observed variation was attributed to alternative splicing of CAMKII.1, a protein kinase that affects transcriptional activity of core clock genes Clock (Clk) and Cycle (Cyc)77. The ecologically relevant role of flexible circadian-controlled gene expression has also been measured in carpenter ants. Here, differences in oscillating gene expression have been implied to play a role in behavioral caste switching. Whole brains of Camponotus floridanus forager ants expressed three times as many genes with a circadian rhythm compared to nurse ant brains. However, many of these 24 h cycling genes in foragers do cycle at the third harmonic (i.e., every 8 h) in nurse brains, and do so in the same phase as the core clock gene period (Per)78. This plasticity in cycling gene expression correlates with, and likely regulates, the difference in circadian locomotor activity between nurse and forager ants. Furthermore, co-expressing genes that were differentially expressed between these behavioral castes, were connected to rhythmic modules in a gene network analysis30, which further strengthens the connection between flexibility in clock-regulation and behavioral plasticity (Fig. 1).
Molecular mechanisms of parasites to manipulate host circadian rhythms
Parasites likely interfere with multiple gene networks as they manipulate a broad set of host behaviors into a stereotyped sequence of events. Computational protein-protein interaction predictions that tested Ophiocordyceps putative effector protein sequences against the carpenter ant host proteome corroborate this79. Overrepresentation analysis of putative binding partners of fungal effectors predicted that ant proteins involved in transcription and DNA binding could be significantly targeted by the fungus. This supports the hypothesis that host behavioral plasticity could indeed be modulated by parasites at the gene regulatory level. However, for this to be plausibly achieved through effector binding, evidence for effector entrance into the host cell and even nucleus would be necessary. This machine learning approach also predicted 33 ant G-protein coupled receptors (GPCRs) as targeted by 16 Ophiocordyceps proteins and peptides in a total of 71 interactions79. Of these receptors, the majority were functionally annotated to bind different neuropeptides and biogenic monoamines such as dopamine and serotonin. Metabolomics data further supports involvement of monoamine neurotransmitters, as these were dysregulated during Ophiocordyceps manipulation80. Another two GPCRs were annotated as ultraviolet and opsin blue light sensitive. Multiple of these GPCRs were also found to be expressed in a circadian manner in carpenter ant brains78. Furthermore, receptors of allatotropin, which is associated with circadian and JH regulation, were predicted to be targeted by manipulation. As such, Ophiocordyceps could manipulate timed behavior by affecting light and ligand sensitivity of these receptors through binding of effectors that either block or activate G-protein pathways. However, how the processes downstream of these GPCRs could give rise to changes in host (timed) behaviors is currently unclear.
Current molecular evidence for parasitic manipulation of host circadian clocks mostly comes from transcriptomics studies. Most rhythmically expressed genes in the whole heads of healthy carpenter ants lost their daily expression pattern about halfway during Ophiocordyceps infection81. Such vast genetic rhythmicity loss could underlie the arrhythmic behavior observed in infected ants at this infection time point19. The rhythmic expression of core clock genes Clk and Per were not among the genes affected. These clock genes were found to co-express in one of three ‘24 h rhythm’ modules of a genome-wide ant gene co-expression network analysis. While this module stayed intact during fungal infection, the other two rhythm modules identified in this network, that contained circadian-regulated genes, significantly lost connectivity81. Therefore, while core clock function in the host ant remains, perhaps to facilitate timed summiting behavior at the end of the infection cycle and/or internal synchrony, the parasite seemingly affects the expression of downstream otherwise rhythmic genes. In turn, this induces the parasite-adaptive arrhythmic phenotypes that help to circumvent social immune responses of the colony. This hypothesis is supported by the finding that many of the same genes that are differentially expressed during fungal manipulation of ant behavior are also differentially expressed between nurse and forager ants30. Moreover, these overlapping behavioral plasticity and manipulated ant genes resided in a network module that was found to be significantly correlated to one of the rhythm modules. This further indicates a parasitic strategy to adaptively manipulate host behavior by hijacking the existing circadian plasticity that facilitates ant caste division.
Nevertheless, it must be noted that circadian rhythms are affected by infections and immune system activation more generally82. Therefore, teasing apart circadian effects specific to host behavior manipulation and those generally induced by infection is necessary. For this purpose, the generalist fungal pathogen Beauveria, which does not adaptively alter its host’s behavior, has proven to be a useful control. Upon Beauveria infection, far less ant host genes lost their rhythmic expression compared to Ophiocordyceps infections. Connectivity in rhythm network modules was also preserved81, and Beauveria-infected ants still showed significant daily activity rhythms19. However, both rhythmic ant host genes and behavioral rhythms demonstrated a phase shift in Beauveria-infected ants compared to healthy controls19,81. This suggests that certain circadian changes in gene expression and behavior are perhaps general hallmarks of disease, while others are infection or manipulation-specific.
Though most specifically studied in Ophiocordyceps-ant interactions so far, examples of molecular clock involvement in host behavioral manipulation have also been reported from other parasite-host interactions. In baculovirus-infected lepidopteran larvae the expression levels of circadian-regulated and core clock genes, including Per and tim, were altered across several ZT time points83. Such differential expression of tim has also been observed in transcriptomics data from Entomophthora-infected flies84. Moreover, knock-out studies demonstrated that viral protein tyrosine phosphatase (PTP) plays a demonstrable role in the induction of hyperactive host behavior by some baculovirus strains85,86. These phosphatases are hypothesized to interact with insect host targets such as foraging, which is linked to light perception and the circadian clock. Additionally, JH levels and takeout, both implicated in circadian regulated (feeding) behavior across insect species87,88, could be targeted by baculovirus PTP85. These PTP targets may in turn regulate neurotransmitter, -peptide and hormone signaling, ultimately resulting in abnormal caterpillar behavior.
Transcriptomics data from Ophiocordyceps-infected ants also suggest a role for parasite PTPs, indicating the potential for convergently evolved molecular mechanisms underlying manipulation phenotypes. Several secreted fungal ptp’s were significantly higher expressed during manipulated summiting behavior20,22. In addition, the expression of one of these fungal ptp’s, as well as other putative behavior-manipulating fungal genes, oscillate with a 24 h rhythm89. This suggests that Ophiocordyceps regulates the production of at least some of its manipulation molecules in a circadian manner. The PTP-target takeout was also downregulated during behavior manipulation in C. floridanus. Similarly, genes involved in JH production and degradation were dysregulated in Ophiocordyceps-infected ants20 and E. muscae-infected flies84, which in E. muscae infections has been linked to the circadian system as discussed above. It is yet to be seen if something similar is happening in fungus-infected ants and baculovirus-infected caterpillars as well. However, current hypotheses allude to this, which suggests that molecular clock processes that underlie behavioral plasticity and manipulation are conserved across insect hosts.
Conclusion
Insect circadian plasticity is manifested across levels of biological organization – from behavioral phenotypes, down to cell circuits and gene expression. Studies investigating the widespread phenomenon of parasitic behavioral manipulation across these levels suggest that this innate circadian plasticity could be exploited to modulate host physiology and behavior in ways that benefit parasite transmission. Timing-related extended phenotypes have been described across independently evolved manipulation systems, suggesting that similar circadian-related mechanisms gave rise to them. For example, both in Ophiocordyceps-infected ants and E. muscae-infected flies, neuropeptidergic and hormonal signals seemingly play key roles in behavior manipulation, while also being the main signaling mode of the circadian clock in general. Interestingly, these parallel findings emerged from analyzing different organizational levels and applying distinct methods. In flies, studies so far have focused on dissecting how different cell populations may communicate to produce the summiting phenotype. In contrast, in ants, different omics technologies were used to identify candidate molecules involved in summiting.
However, it comes to no surprise that results across independent experiments, labs and manipulation systems show some level of congruence. Comparative studies of the molecular clock in general have found similar clock genes and feedback loop principles conserved across the animal kingdom. Following the pioneering study in flies that identified the first circadian gene Per, immunohistochemical studies have identified Per+ central pacemakers in other insects, including cockroaches, crickets, beetles, moths and eusocial insects like honeybees and ants43,57,90,91. Overall cellular organizations and circadian oscillations of clock gene expression and protein level are similar. Though species-specific differences that may have evolved to meet differences in ecological needs, also exist57. As such, parasite taxa that are on independent evolutionary trajectories towards host behavioral manipulation as a strategy to increase fitness are likely presented with similar selection pressures and opportunities to overcome them. Comparable host circadian rhythms would present similar selection pressures to which parasites must adapt, and circadian plasticity would provide a similar backdoor into hijacking them, leading to convergent parasite mechanisms. Such taxa-wide convergence in infection mechanisms is also seen in other aspects of parasite-host interactions besides manipulation92. Therefore, unraveling what role circadian clock plasticity plays in one manipulative parasite-host system might provide testable hypotheses for others and push the entire research field forward. Moreover, these manipulation systems with clear rhythmic disease phenotypes provide an opportunity to better understand the intersection between biological clocks and infectious diseases.
Taken together, we advocate for more comparative studies across manipulation systems and integration of the largely separated fields of chrono-/neurobiology, infection biology and behavioral ecology, to achieve a comprehensive understanding of behavior-manipulation mechanisms. Ecological field studies provide the opportunity to observe, record, and place (altered) rhythmic phenotypes into evolutionary context. However, such studies are prone to environmental variability that possibly masks biological effects and complicates addressing mechanistic questions. In contrast, molecular work explores biological questions by targeting detailed cellular and molecular processes in controlled conditions but typically lacks a discussion on the functional ecological relevance of these mechanisms. The trade-off between ecological relevance and mechanistic understanding may be overcome by applying various –omics technologies and functional studies under laboratory settings that carefully mimic natural conditions informed by ecological surveys. Many molecular techniques can be used with high cellular resolution, e.g. sequencing across single cells in a tissue. Such resolution may prove useful in future studies of neural mechanisms of behavior-manipulation, considering that only specific clock sub-populations appear relevant in manipulated flies67 and that the clock cellular network is highly heterogenous in general. Analyzing the cellular diversity of clock neurons will be important in the future for uncovering its functional relevance to generate circadian plasticity across different ecological contexts. The natural experiment that behavior-manipulating parasites provide might prove useful in this endeavor while unraveling the cells and genes involved in parasite-induced changes in host rhythmicity will in turn reveal their functions under normal circadian conditions.
Data availability
No datasets were generated or analyzed during the current study.
References
Eelderink-Chen, Z. et al. A circadian clock in a nonphotosynthetic prokaryote. Sci. Adv. 7, https://doi.org/10.1126/sciadv.abe2086 (2021).
Dodd, A. N. et al. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 309, 630–633 (2005).
Hunter, F. K., Butler, T. D. & Gibbs, J. E. Circadian rhythms in immunity and host-parasite interactions. Parasite Immunol. 44, e12904 (2022).
Patke, A., Young, M. W. & Axelrod, S. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 21, 67–84 (2020).
Rund, S. S., O’Donnell, A. J., Gentile, J. E. & Reece, S. E. Daily rhythms in mosquitoes and their consequences for malaria transmission. Insects 7, https://doi.org/10.3390/insects7020014 (2016).
Rund, S. S. C., O’Donnell, A. J., Prior, K. F. & van der Veen, D. R. Seasonal plasticity in daily timing of flight activity in Anopheles stephensi is driven by temperature modulation of dawn entrainment. Philos. Trans. R. Soc. Lond. B Biol. Sci. 380, 20230343 (2025).
Oke, C. E. et al. Biting time of day in malaria mosquitoes is modulated by nutritional status. bioRxiv https://doi.org/10.1101/2025.04.28.650966 (2025).
O’Donnell, A. J., Rund, S. S. C. & Reece, S. E. Time-of-day of blood-feeding: effects on mosquito life history and malaria transmission. Parasit. Vectors 12, 301 (2019).
Hut, R. A., Pilorz, V., Boerema, A. S., Strijkstra, A. M. & Daan, S. Working for food shifts nocturnal mouse activity into the day. PLoS One 6, e17527 (2011).
van Rosmalen, L. et al. Energy balance drives diurnal and nocturnal brain transcriptome rhythms. Cell Rep. 43, 113951 (2024).
Schulz, D. J., Vermiglio, M. J., Huang, Z. Y. & Robinson, G. E. Effects of colony food shortage on social interactions in honey bee colonies. Insectes Sociaux 49, 50–55 (2002).
Druiven, S. J. M. et al. Chronotype changes with age; seven-year follow-up from the Netherlands study of depression and anxiety cohort. J. Affect. Disord. 295, 1118–1121 (2021).
Gronenberg, W., Heeren, S. & Hölldobler, B. Age-dependent and task-related morphological changes in the brain and the mushroom bodies of the ant Camponotus floridanus. J. Exp. Biol. 199, 2011–2019 (1996).
Gordon, D. M. Dynamics of task switching in harvester ants. Anim. Behav. 38, 194–204 (1989).
Hawking, F. Circadian and other rhythms of parasites. Adv. Parasitol. 13, 123–182 (1975).
Moulia-Pelat, J. P. et al. Periodicity of Wuchereria bancrofti var. pacifica filariasis in French Polynesia. Trop. Med. Parasitol. 44, 83–85 (1993).
Pigeault, R., Caudron, Q., Nicot, A., Rivero, A. & Gandon, S. Timing malaria transmission with mosquito fluctuations. Evol. Lett. 2, 378–389 (2018).
Jones, J. D. G. & Dangl, J. L. The plant immune system. Nature 444, 323–329 (2006).
Trinh, T., Ouellette, R. & de Bekker, C. Getting lost: the fungal hijacking of ant foraging behaviour in space and time. Anim. Behav. 181, 165–184 (2021).
Will, I. et al. Genetic underpinnings of host manipulation by ophiocordyceps as revealed by comparative transcriptomics. G3 Genes Genomes Genet. 10, 2275–2296 (2020).
Van Roosmalen, E. & De Bekker, C. Mechanisms underlying Ophiocordyceps infection and behavioural manipulation of ants: Unique or ubiquitous? Annu. Rev. Microbiol. 78, 575−593 (2024).
de Bekker, C. et al. Gene expression during zombie ant biting behavior reflects the complexity underlying fungal parasitic behavioral manipulation. BMC Genomics 16, 620 (2015).
Hughes D. P. et al. Behavioral mechanisms and morphological symptoms of zombie ants dying from fungal infection. BMC Ecol. 11, https://doi.org/10.1186/1472-6785-11-13 (2011).
Krasnoff, S. B., Watson, D. W., Gibson, D. M. & Kwan, E. C. Behavioral effect of the Entomopathogenic Fungus, Entomophthora muscae on its Host A4usca domestica: postural changes in dying hosts and gated pattern of mortality. J. Insect Physiol. 41, 895–903 (1995).
Kramer, J. P. Entomophthora muscae — moisture as a factor affecting its transmission and conidial germination. Acta Mycologica 16, 1 (1980).
Will, I., Linehan, S., Jenkins, D. G. & de Bekker, C. Natural history and ecological effects on the establishment and fate of Florida carpenter ant cadavers infected by the parasitic manipulator Ophiocordyceps camponoti-floridani. Funct. Ecol. 37, 886–899 (2023).
Andriolli, F. S. et al. Do zombie ant fungi turn their hosts into light seekers?. Behav. Ecol. 30, 609–616 (2019).
Loreto, R. G., Elliot, S. L., Freitas, M. L., Pereira, T. M. & Hughes, D. P. Long-term disease dynamics for a specialized parasite of ant societies: a field study. PLoS One 9, e103516 (2014).
Brobyn, P. J. & Wilding, N. Invasive and developmental processes of Entomophthora muscae infecting houseflies (Musca domestica). Trans. Br. Mycological Soc. 80, 1–8 (1983).
de Bekker, C. & Das, B. Hijacking time: how Ophiocordyceps fungi could be using ant host clocks to manipulate behavior. Parasite Immunol. 44, e12909 (2022).
Goulson, D. Wipfelkrankheit: modification of host behaviour during baculoviral infection. Oecologia 109, 219–228 (1997).
Casey, T. M. Activity patterns, body temperature and thermal ecology in two desert caterpillars (Lepidoptera: Sphingidae). Ecology 57, 485–497 (1976).
Leonard, D. E. Feeding Rhythm in larvae of the Gypsy Moth. J. Econ. Entomol. 63, 1454–1457 (1970).
Hoover, K. et al. A gene for an extended phenotype. Science 333, 1401 (2011).
Han, Y., van Houte, S., van Oers, M. M. & Ros, V. I. Timely trigger of caterpillar zombie behaviour: temporal requirements for light in baculovirus-induced tree-top disease. Parasitology 145, 822–827 (2017).
Han, Y., van Houte, S., Drees, G. F., van Oers, M. M. & Ros, V. I. D. Parasitic manipulation of host behaviour: Baculovirus SeMNPV EGT facilitates tree-top disease in spodoptera exigua larvae by extending the time to death. Insects 6, 716–731 (2015).
Ponton, F. et al. Water-seeking behavior in worm-infected crickets and reversibility of parasitic manipulation. Behav. Ecol. 22, 392–400 (2011).
Sawada, Y. et al. A potential evolutionary trap for the extended phenotype of a nematomorph parasite. PNAS Nexus 3, pgae464 (2024).
Biron, D. G. et al. Behavioural manipulation in a grasshopper harbouring hairworm: a proteomics approach. Proc. Biol. Sci. 272, 2117–2126 (2005).
Obayashi, N. et al. Enhanced polarotaxis can explain water-entry behaviour of mantids infected with nematomorph parasites. Curr. Biol. 31, R777–R778 (2021).
Moore, J. An overview of parasite-induced behavioral alterations - and some lessons from bats. J. Exp. Biol. 216, 11–17 (2013).
Reinhard, N. et al. Synaptic connectome of the Drosophila circadian clock. Nat. Commun. 15, 10392 (2024).
Helfrich-Förster, C. Robust circadian rhythmicity of Drosophila melanogaster requires the presence of lateral neurons: a brain-behavioral study of disconnected mutants. J. Comp. Physiol. A 182, 435–453 (1998).
Dorkenwald, S. et al. Neuronal wiring diagram of an adult brain. Nature 634, 124–138 (2024).
Ma, D. et al. Transcriptomic DN3 clock neuron subtypes regulate Drosophila sleep. Sci. Adv. 11, eadr4580 (2025).
Ma, D. et al. A transcriptomic taxonomy of Drosophila circadian neurons around the clock. eLife 10, https://doi.org/10.7554/elife.63056 (2021).
Lamaze, A. & Stanewsky, R. DN1p or the “Fluffy” cerberus of clock outputs. Front. Physiol. 10, 1540 (2020).
Dubowy, C. & Sehgal, A. Circadian rhythms and sleep in Drosophila melanogaster. Genetics 205, 1373–1397 (2017).
Reinhard, N. et al. The neuronal circuit of the dorsal circadian clock neurons in Drosophila melanogaster. Front. Physiol. 13, 1–29 (2022).
Helfrich-Forster, C. & Reinhard, N. Mutual coupling of neurons in the circadian master clock: what we can learn from fruit flies. Neurobiol. Sleep. Circadian Rhythms 18, 100112 (2025).
Sheng, Z., Xu, J., Bai, H., Zhu, F. & Palli, S. R. Juvenile hormone regulates vitellogenin gene expression through insulin-like peptide signaling pathway in the red flour beetle, Tribolium castaneum. J. Biol. Chem. 286, 41924–41936 (2011).
Truman, J. W. et al. Juvenile hormone is required to couple imaginal disc formation with nutrition in insects. Science 312, 1385–1388 (2006).
Zhang, X., Li, S. & Liu, S. Juvenile hormone studies in Drosophila melanogaster. Front. Physiol. 12, https://doi.org/10.3389/fphys.2021.785320 (2022).
Ju, L. et al. Hormonal gatekeeping via the blood-brain barrier governs caste-specific behavior in ants. Cell 186, 4289–4309.e4223 (2023).
Glastad, K. M. et al. Epigenetic regulator CoREST controls social behavior in ants. Cell 77, 338–351.e336 (2020).
McKim, T. H. et al. Synaptic connectome of a neurosecretory network in the Drosophila brain. eLife https://doi.org/10.7554/eLife.102684.1.sa4 (2024).
Beer, K. & Helfrich-Forster, C. Model and non-model insects in chronobiology. Front. Behav. Neurosci. 14, 601676 (2020).
Carvalhas-Almeida, C. & Sehgal, A. Glia: the cellular glue that binds circadian rhythms and sleep. Sleep 48, https://doi.org/10.1093/sleep/zsae314 (2025).
Hastings, M. H., Brancaccio, M., Gonzalez-Aponte, M. F. & Herzog, E. D. Circadian rhythms and astrocytes: the good, the bad, and the ugly. Annu. Rev. Neurosci. 46, 123–143 (2023).
Ewer, J., Frisch, B., Hamblen-Coyle, M. J., Rosbash, M. & Hall, J. C. Expression of the period clock gene within different cell types in the brain of Drosophila adults and mosaic analysis of these cells’ influence on circadian behavioral rhythms. J. Neurosci. 12, 3321–3349 (1992).
Dopp, J. et al. Single-cell transcriptomics reveals that glial cells integrate homeostatic and circadian processes to drive sleep-wake cycles. Nat. Neurosci. 27, 359–372 (2024).
Zhang, S. L., Zhifeng, Y., Arnold, D. M., Artiushin, G. & Sehgal, A. A circadian clock in the blood-brain barrier regulates xenobiotic efflux. Cell 173, 130–139 (2018).
Li, Y., Haynes, P., Zhang, S. L., Yue, Z. & Sehgal, A. Ecdysone acts through cortex glia to regulate sleep in Drosophila. eLife 12, https://doi.org/10.7554/eLife.81723 (2023).
Vaughen, J. P. et al. Glial control of sphingolipid levels sculpts diurnal remodeling in a circadian circuit. Neuron 110, 3186–3205 (2022).
Rijo-Ferreira, F. et al. The malaria parasite has an intrinsic clock. Science 368, 746–753 (2020).
Rijo-Ferreira, F. & Takahashi, J. S. Sleeping sickness: a tale of two clocks. Front. Cell Infect. Microbiol. 10, 525097 (2020).
Elya, C. et al. Neural mechanisms of parasite-induced summiting behavior in ‘zombie’ Drosophila. Elife 12, https://doi.org/10.7554/eLife.85410 (2023).
Fredericksen, M. A. et al. Three-dimensional visualization and a deep-learning model reveal complex fungal parasite networks in behaviorally manipulated ants. PNAS 114, 12590–12595 (2017).
Mangold, C. A., Ishler, M. J., Loreto, R. G., Hazen, M. L. & Hughes, D. P. Zombie ant death grip due to hypercontracted mandibular muscles. J. Exp. Biol. 222, https://doi.org/10.1242/jeb.200683 (2019).
Liang, X., Holy, T. E. & Taghert, P. H. Synchronous Drosophila circadian pacemakers display nonsynchronous Ca2+ rhythms in vivo. Science 351, 976–981 (2016).
Wen, S. et al. Spatiotemporal single-cell analysis of gene expression in the mouse suprachiasmatic nucleus. Nat. Neurosci. 23, 456–467 (2020).
Ma, D. et al. Timeless noncoding DNA contains cell-type preferential enhancers important for proper Drosophila circadian regulation. Proc. Natl. Acad. Sci. USA 121, e2321338121 (2024).
Tauber, E. et al. Natural selection favors a newly derived timeless allele in Drosophila melanogaster. Science 316, 1895–1898 (2007).
Sandrelli, F. et al. A molecular basis for natural selection at the timeless Locus in Drosophila melanogaster. Science 316, 1898–1900 (2007).
Lamaze, A. et al. A natural timeless polymorphism allowing circadian clock synchronization in “white nights. Nat. Commun. 13, 1724 (2022).
Shahandeh, M. P. et al. Circadian plasticity evolves through regulatory changes in a neuropeptide gene. Nature 635, 951–959 (2024).
Kaiser, T. S. et al. The genomic basis of circadian and circalunar timing adaptations in a midge. Nature 540, 69–73 (2016).
Das, B. & de Bekker, C. Time-course RNASeq of Camponotus floridanus forager and nurse ant brains indicate links between plasticity in the biological clock and behavioral division of labor. BMC Genomics 23, 57 (2022).
Will, I., Beckerson, W. C. & de Bekker, C. Using machine learning to predict protein-protein interactions between a zombie ant fungus and its carpenter ant host. Sci. Rep. 13, 13821 (2023).
Will, I., Attardo, G. M. & de Bekker, C. Multiomic interpretation of fungus-infected ant metabolomes during manipulated summit disease. Sci. Rep. 13, 14363 (2023).
Das, B., Brachmann, A. & de Bekker, C. Both behavior-manipulating and non-manipulating entomopathogenic fungi affect rhythmic gene expression in carpenter ant foragers upon infection. bioRxiv https://doi.org/10.1101/2023.01.19.524837 (2023).
Wang, C., Lutes, L. K., Barnoud, C. & Scheiermann, C. The circadian immune system. Sci. Immunol. 7, eabm2465 (2022).
Bhattarai, U. R., Li, F., Katuwal Bhattarai, M., Masoudi, A. & Wang, D. Phototransduction and circadian entrainment are the key pathways in the signaling mechanism for the baculovirus induced tree-top disease in the lepidopteran larvae. Sci. Rep. 8, 17528 (2018).
Elya, C. et al. Robust manipulation of the behavior of Drosophila melanogaster by a fungal pathogen in the laboratory. Elife 7, https://doi.org/10.7554/eLife.34414 (2018).
van Houte, S. et al. Protein tyrosine phosphatase-induced hyperactivity is a conserved strategy of a subset of baculoviruses to manipulate lepidopteran host behavior. PLoS One 7, e46933 (2012).
Kamita, S. G. et al. A baculovirus-encoded protein tyrosine phosphatasegene induces enhanced locomotory activity in alepidopteran host. PNAS 102, 2585–2589 (2004).
Das, S. & Dimopoulos, G. Molecular analysis of photic inhibition of blood-feeding in Anopheles gambiae. BMC Physiol. 8, https://doi.org/10.1186/1472-6793-8-23 (2008).
Sarov-Blat, L., So, W. V., Liu, L. & Rosbash, M. The Drosophila takeout gene is a novel molecular link between circadian rhythms and feeding behavior. Cell 101, 647–656 (2000).
de Bekker, C., Will, I., Hughes, D. P., Brachmann, A. & Merrow, M. Daily rhythms and enrichment patterns in the transcriptome of the behavior-manipulating parasite Ophiocordyceps kimflemingiae. PLoS One 12, e0187170 (2017).
Kay, J., Menegazzi, P., Mildner, S., Roces, F. & Helfrich-Forster, C. The circadian clock of the ant camponotus floridanus is localized in dorsal and lateral neurons of the brain. J. Biol. Rhythms 33, 255–271 (2018).
Beer, K. et al. Pigment-dispersing factor-expressing neurons convey circadian information in the honey bee brain. Open Biol. 8, https://doi.org/10.1098/rsob.170224 (2018).
Perlin, M. H., Poulin, R. & de Bekker, C. Invasion of the four kingdoms: the parasite journey across plant and non-plant hosts. Biol. Rev. Camb. Philos. Soc. https://doi.org/10.1111/brv.13169 (2024).
Acknowledgements
This work is supported by ERC grant ZOMBIHAVIOUR, https://doi.org/10.3030/101124277. Views and opinions expressed are however those of the authors only and do not necessarily reflect those of the European Union or the European Research Council Executive Agency. Neither the European Union nor the granting authority can be held responsible for them.
Author information
Authors and Affiliations
Contributions
J.D. and C.B. conceptualized, wrote, and edited the manuscript. J.D. prepared the accompanying figure. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dopp, J., de Bekker, C. Insect circadian plasticity as a proposed target for the expression of parasite extended phenotypes. npj Biol Timing Sleep 2, 29 (2025). https://doi.org/10.1038/s44323-025-00046-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44323-025-00046-0