Abstract
Glucose 6-phosphate dehydrogenase deficiency (G6PD-d) is the most common enzymopathy in the world, occurring in 5–8% of the global population (half a billion people). Recent epidemiological evidence suggests that G6PD-d may be associated with increased cardiovascular disease (CVD). Atherosclerosis is the dominant cause of CVD, including myocardial infarction, heart failure, stroke, and peripheral artery disease. Atherosclerosis, in turn, is a chronic inflammatory disease, fueled by oxidized lipids and influenced by various immune and nonimmune cells including vascular endothelial and smooth muscle cells, monocytes and macrophages, T cells, B cells, and red blood cells. Here, we review the existing epidemiological evidence supporting a role for G6PD-d in CVD in humans and explore the data on potential cellular mechanisms by which G6PD-d may exacerbate atherosclerosis.
Similar content being viewed by others
Introduction
Cardiovascular disease (CVD) is the leading cause of mortality and morbidity worldwide1. A major cause of CVD is atherosclerosis, a chronic inflammatory disease characterized by the pathogenic accumulation of vascular smooth muscle cells (VSMC), oxidized lipids and immune cells in the artery wall2,3,4. Atherosclerosis is a disorder with multiple environmental and genetic contributions. Incorporation of genetic variants identified through genome wide association and CVD cohort studies has successfully refined CVD risk prediction models indicating that heritable genetic variants play an integral role in CVD pathogenesis and progression. Thus, identifying CVD-associated variants may inform precision medicine efforts to prevent and treat CVD5,6.
Recent epidemiological studies have identified the glucose 6-phosphate dehydrogenase (G6PD) gene on the X chromosome as one possible location of such genetic variants. G6PD deficiency (G6PD-d) is the most common enzymopathy in the world, with approximately 500 million affected people worldwide. The impressive prevalence of G6PD variants is hypothesized to be related to their conferred resistance to malaria. These mutations therefore disproportionately affect individuals with ancestry from malaria endemic regions, namely Africa, the Middle East, Southeast Asia, and the Mediterranean. Affected individuals display a spectrum of disease severity corresponding to the degree of reduction in G6PD activity, which is determined by the specific G6PD variant alleles present7,8,9. X-inactivation in female heterozygotes further increases phenotypic variability10. Importantly, most amino acid-altering mutations that reduce the overall activity of G6PD do so by decreasing protein stability, catalytic capacity or both. Accordingly, the World Health Organization classifies G6PD-d based on the level of residual G6PD activity, with severe deficiency (Class I) causing chronic nonspherocytic hemolytic anemia and the least severe deficiency (Class IV) with little-to-no appreciable phenotype8,11.
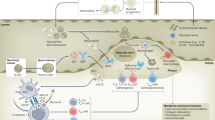
G6PD plays an essential role in redox homeostasis by catalyzing the first and rate limiting step of the pentose phosphate pathway (PPP), producing a significant portion of the cellular store of nicotinamide adenine dinucleotide phosphate (NADPH). NADPH is the primary electron donor for multiple antioxidant pathways. For example, NADPH is the electron donor for the enzymatic regeneration of the reduced form of the important antioxidant glutathione (GSH), enabling it to neutralize toxic reactive oxygen species (ROS) that accumulate due to normal and pathological cellular processes. Additionally, NADPH is a necessary cofactor for fatty acid, amino acid, and nucleotide biosynthesis. The PPP also produces ribose-5-phosphate required for ribonucleotide synthesis12 (Fig. 1a).
a G6PD is the rate-limiting enzyme in the pentose phosphate pathway (PPP), producing NADPH and other biosynthetic molecules necessary for the synthesis of ribonucleic acids, fatty acids, nucleotides, and amino acids. NADPH produced by the PPP is critical for maintaining redox homeostasis and, conversely, for the production of reactive oxygen species by NADPH oxidases. b, c G6PD deficiency is associated with worsened cardiovascular disease outcomes and a higher incidence of immune-related diseases in epidemiological studies. However, the effect of G6PD-d and a dysfunctional PPP on immune cell function in the context of atherosclerosis has yet to be explored.
Traditionally, G6PD-d has been studied in the context of RBC hemolysis secondary to ingestion of drugs or foods that cause oxidative stress. RBCs are particularly susceptible to oxidative stress due to the lack of nuclei needed for protein synthesis, the absence of mitochondria capable of supplying NADPH outside of the PPP, and a high iron content that drives Fenton chemistry8. However, a growing body of evidence points to a role for G6PD-d in pathologies other than hemolysis, including atherosclerotic cardiovascular disease (ASCVD). (Fig. 1b) Interestingly, reduced G6PD activity has also been implicated in dysfunctional innate and adaptive immune responses13,14. (Fig. 1c) That ASCVD is a chronic inflammatory disease mediated by both the innate and adaptive immune systems, provides potential mechanisms whereby G6PD-d may contribute to atherosclerosis2. In this review, we highlight the epidemiological evidence linking G6PD-d to ASCVD and discuss the literature that supports mechanisms that may explain this link, with a focus on the potential impact of G6PD-d on the immune cells involved in atherosclerosis.
Epidemiological evidence of the link between G6PD & CVD
Early epidemiological studies of G6PD-d and atherosclerosis suggested that it may be atheroprotective. Long et al. found a significant reduction in the incidence of coronary artery disease among African American individuals with G6PD-d. However, the study did not control for variation in cholesterol levels, which is a major cardiovascular disease covariate. Additionally, G6PD deficiency was assessed by the methemoglobin reduction test, which has a low positive predictive value and therefore may classify individuals as being G6PD-d when they are not15. A subsequent study by Cocco et al. found a decreased risk of mortality caused by ischemic heart disease and cerebrovascular disease in G6PD-d individuals, although the study was underpowered by limited sample sizes16. Meloni et al. reported a decreased incidence of G6PD-d in patients presenting to the hospital for coronary artery disease, though the sample size of G6PD-d patients was again limited and the case control design has more limitations when compared to retrospective cohort studies17.
More recently, studies with larger sample sizes and superior study design have demonstrated a significantly increased risk of ASCVD associated with G6PD-d. In 2018, a study of 737 G6PD-d individuals in the US Armed Forces demonstrated a covariate-adjusted risk of ASCVD that was 39.6% higher than non-deficient controls18. Similarly, Pes et al. published a propensity score-matched study of 1123 Mediterranean G6PD-d subjects, the largest sample size of G6PD-d subjects to date, which showed a 71% higher risk of ASCVD after controlling for available ASCVD-related covariates19. Two more recent studies investigating the risk of stroke in G6PD-d subjects in China concluded that G6PD-d was associated with significantly increased risk of large vessel atherosclerosis, stroke history, and intracranial atherosclerotic stenosis (ICAS)20,21. While Dore et al. found that the risk of ASCVD complications was only statistically significant in individuals over the age of 60 and Li et al. found that G6PD-d increases risk of ICAS in those aged 70 years or older, others studies have noted increased ASCVD risk in populations ranging from ~37 to 51 years old18,19,20,21. This potential age-related effect may be secondary to a progressive imbalance between ROS production and waning antioxidant defenses during aging, making G6PD-d individuals more susceptible to oxidative stress-induced ASCVD22. ASCVD is also a chronic disease that develops across the lifespan, meaning G6PD-d individuals may accumulate subclinical atherosclerotic plaque at an accelerated rate prior to age 60. After enough time, their risk for the symptomatic ASCVD outcomes (i.e. MI, stroke) measured in these cohort studies may be increased. However, further studies with more quantitative measures of ASCVD are needed to assess this possibility.
Lastly, epidemiological data suggests G6PD-d may modify several cardiovascular risk factors that can contribute to the development of ASCVD. While the impact of G6PD-d on these covariates has been discussed thoroughly in a previous review, we will summarize here for completeness23. In short, G6PD-d increases the risk of developing chronic hypertension, diabetes, and the microvascular complications of diabetes, which are all major risk factors for ASCVD18,24,25,26,27. Interestingly, several in vitro studies of experimentally induced G6PD-d in human monocytes and endothelial cells have demonstrated that exposure to hyperglycemia or free fatty acids alone, as seen in diabetes or hyperlipidemia, decrease G6PD activity. Thus, these comorbid conditions may further exacerbate the proinflammatory endothelial and monocyte phenotypes associated with preexisting G6PD-d, thereby compounding CVD risk28,29,30.
Mechanistic studies linking G6PD-d to cellular processes involved in atherosclerosis
G6PD-d impairs vascular homeostasis
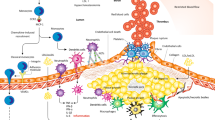
Nitric oxide (NO) plays an important role in the early development of ASCVD. NO inhibits leukocyte adhesion to vascular endothelial cells (VEC) in vitro and in vivo during the early phases of hypercholesterolemia31,32. In addition, NO regulates low density lipoprotein (LDL) levels and vascular uptake. NO normally functions to reduce plasma LDL concentration via S-nitrosylation of the LDL receptor adaptor protein ARH, thereby enhancing LDL receptor-mediated clearance of LDL33. In the arterial wall, NO promotes vasodilation and maintains the integrity of the vascular endothelial barrier, thereby limiting the harmful translocation of LDL to the arterial intima34 (Fig. 2a).
G6PD-d reduces the production of cellular NADPH. a NADPH is a necessary cofactor for nitric oxide (NO) synthesis by endothelial nitric oxide synthase (eNOS). G6PD-d therefore decreases bioavailable NO, potentially leading to vasoconstriction, increased vascular permeability, increased leukocyte adhesion, vascular smooth muscle cell (VSMC) proliferation, and reduced LDL clearance through insufficient NO-modification of LDL receptor (LDLR) adaptor protein ARH. b NADPH is also a necessary cofactor for the generation of glutathione (GSH), which is a key antioxidant. Decreased GSH leads to increased ROS. This may lead to vascular endothelial cell (VEC) damage and death, pro-inflammatory cytokine secretion, and expression of leukocyte adhesion molecules that recruit inflammatory immune mediators of atherosclerosis. ROS accumulation may also contribute to increased oxidation of low density lipoprotein (LDL) into oxLDL, which is inflammatory and pathogenic in ASCVD. c Interleukin 10 (IL-10) production by monocytes and other immune cells may be decreased in humans with G6PD-d, though this may be due to the presence of low-producing IL-10 variants that commonly co-occur with G6PD-d variant alleles. Regardless of why G6PD-d individuals express lower IL-10 levels, reduced IL-10 may lead to increased atherosclerosis as it plays a predominantly anti-inflammatory role in ASCVD.
Nitric oxide synthase (NOS) requires NADPH as a cofactor to produce NO. Given G6PD produces a majority of cellular NADPH, G6PD-d cells demonstrate impaired NO production in vitro35. Studies of cultured human vascular endothelial cells (HUVECs) have demonstrated that competitive inhibition of G6PD in the HUVECs using 6-aminonicotinamide (6-AN) or G6PD knockdown downregulate eNOS activity and nitrate levels. Despite G6PD-d classically leading to reduced NADPH bioavailability, NADPH oxidase-dependent ROS production was also increased28,29. Though not examined in this study, NO has been shown to inhibit NOX activity through s-nitrosylation. This effect of NO depletion may represent an additional mechanism by which G6PD enhances ROS production in G6PD-d cells. The G6PD-d HUVECs also demonstrated increased expression of VCAM/ICAM-1, TGFβ and other pro-inflammatory molecules such as TNF and MCP-1. When co-cultured with a human-derived monocyte line with normal G6PD activity, HUVECs rendered G6PD-d via inhibition with 6-AN, dehydroepiandosterone (DHEA), or G6PD knockdown displayed increased monocyte adhesion. Notably, inhibition of NOXs or TGFβ receptors significantly decreased monocyte adhesion to the G6PD-d HUVECs. The authors of these studies therefore concluded that the observed proinflammatory phenotype is likely mediated by increased TGFβ/NOX/ROS signaling within the HUVECs23,30. Altogether, these proinflammatory changes in vascular homeostasis are known to promote proatherogenic accumulation of both inflammatory leukocytes and LDL in the vascular wall36 (Fig. 2b).
In addition to its effects on the VECs, G6PD-d may contribute to plaque growth by increasing the proliferative capacity of VSMCs and reducing vasodilation37,38. Although contrary to the notion that G6PD-d largely limits proliferative capacity, Varghese et al. found that G6PD-d mice spontaneously developed pulmonary hypertension secondary to increased VSMC proliferation. This proliferative phenotype was shown to be induced by ROS/RNS-dependent PI3K, ERK1/2, and AMPK activation37. Other groups have also reported that G6PD-d VSMCs may have increased expression of Il-6, as well as various chemokines and chemokine receptors that can potentiate vascular inflammation38,39. Furthermore, reduction in bioavailable NO induces pathogenic vascular smooth muscle cell proliferation in vitro and vasoconstriction in vivo due to suppression of cGMP kinase signaling40,41. While high sheer stress caused by vasoconstriction is considered atheroprotective during the early phases of atherogenesis, it later predisposes individuals to more vulnerable plaque phenotypes that cause MI and stroke42. VSMC proliferation and intimal thickening also predispose individuals to oscillatory or low blood flow, which have been shown to induce leukocyte adhesion, enhanced vascular permeability, LDL oxidation, and atherosclerotic plaque formation42,43,44. (Fig. 2a) It is important to note that there is also evidence to the contrary that suggests G6PD-d may exert a vasoprotective effect by preventing pathogenic VSMC proliferation, limiting vasoconstriction, and protecting against angiotensin II-induced hypertension45,46. Given this is an area of active research, more studies are needed to fully elucidate the impact of G6PD-d on VSMCs in atherosclerosis.
G6PD-d impairs antioxidant processes
In individuals with sufficient G6PD activity, GSH neutralizes ROS to maintain redox homeostasis. However, in G6PD-d individuals with depleted GSH stores, LDL oxidation is hypothesized to be accelerated by the resulting oxidative stress. Consistent with this model, serum samples from G6PD-d patients have been reported to have significant increases in oxidation specific epitopes (OSE) [i.e. a 31% increase in a product of lipid oxidation (MDA) and a 447% increase in a product of protein oxidation (protein carbonyl)]47. In atherogenesis, pattern recognition receptors (PRRs) and scavenger receptors on intimal macrophages are known to recognize these OSEs as damage associated pathogens (DAMPs). Upon recognition, macrophages undergo proinflammatory polarization and phagocytose of oxidized low density lipoprotein (oxLDL), ultimately leading to proatherogenic cytokine production and the formation of foam cells that contribute to plaque progression2 (Fig. 2b).
G6PD-d and IL-10
In several animal models of ASCVD, IL-10 has been shown to reduce plaque burden and improve plaque phenotype while ApoE-/- IL10-/- double knockout mice develop increased atherosclerosis48. Human peripheral blood mononuclear cells (PBMC) from G6PD-d patients harboring G6PD variants have demonstrated reduced IL-10 secretion in response to stimulation49,50,51. However, several animal and human studies have cast doubt on the effect of G6PD-d on IL-10 secretion. PBMCs from G6PD-d infants showed no difference in IL-10 secretion compared to controls following toll-like receptor (TLR) stimulation52. Similarly, other in vitro studies of G6PD-d mouse demonstrate no difference in the production of IL-10 by macrophages53,54. Upperman ii identified a significantly higher frequency of low-producing IL-10 genotypes in G6PD-d individuals that may explain the discrepancy between the results from human and mouse studies55. (Fig. 2c) Therefore, while more mouse studies are needed, IL-10 may represent a confounding variable in human studies of G6PD-d in ASCVD.
Role of G6PD-deficiency-mediated RBC hemolysis in ASCVD
Any discussion of the potential impact of G6PD-d on ASCVD would be incomplete without considering how the predominant hemolytic phenotype may contribute to worsened disease. Although most individuals with G6PD variant alleles are asymptomatic with no detectable hemolysis at baseline, RBCs do accumulate oxidative damage at an accelerated rate over time11. Destruction of oxidatively-damaged RBCs is accomplished extravascularly by macrophages in the reticuloendothelial system56. However, upon exposure to powerful pro-oxidant stimuli such as dapsone or primaquine, acute extravascular and then intravascular hemolysis can occur leading to the release of heme and free iron into the local intravascular environment57. Hyperlipidemia is a prevalent source of oxidative stress that has been shown to increase red cell fragility and induce hemolysis, though the extent to which it may induce chronic low-grade intravascular hemolysis in a G6PD-d model is uncertain58,59.
In the context of intravascular hemolysis, free iron released locally can directly produce ROS through participation in Fenton reactions. Free heme is known to scavenge NO, potentially exacerbating the NO deficiency observed in G6PD-d and leading to vasoconstriction, increased vascular permeability, uptake of LDL, immune cell recruitment, and accelerated oxidation of LDL60. Additionally, free heme can generate the reactive nitrogen species peroxynitrite that can further oxidative stress61. (Fig. 3a) Consistent with a deleterious role for free iron in ASCVD, ApoE-/- mice exposed to chronic iron overload displayed exacerbated atherosclerosis likely secondary to VEC damage or death, which was attenuated by dietary iron restriction or iron chelation62,63.
G6PD-d can lead to both intravascular and extravascular red blood cell (RBC) destruction. a Although minimal, acute intravascular hemolysis can occur when G6PD-d individuals consume pro-oxidant medications or potentially due to chronic pro-oxidant stressors such as hyperlipidemia. Hemolysis releases heme and iron into the intravascular space. Free heme can chelate NO, thereby reducing bioavailable NO, and can directly generate powerful reactive nitrogen species (ONOO-, peroxynitrite). Together, these can worsen VEC oxidative stress as well as the NO-depleted phenotype previously discussed (vasoconstriction, vascular permeability, leukocyte adhesion, VSMC proliferation, LDL clearance). b Extravascular destruction of oxidatively-damaged RBCs by the reticuloendothelial system accounts for a majority of RBC destruction in G6PD-d. Increased vascular permeability due to NO-depletion may increase RBC infiltration into the vascular endothelium where RBCs are further oxidized (oxRBC). Macrophage (Mφ) consumption of oxRBCs and subsequent iron overload may lead to foam cell formation as well as inflammatory Mφ formation with associated proinflammatory cytokine secretion. c Free iron exposure (VEC) and oxRBC consumption may increase cellular propensity for ferroptosis. Ferroptosis is an iron-dependent form of cell death, mediated by the formation of polyunsaturated fatty acid (PUFA) lipid peroxides, that has been linked to atherogenesis. Additionally, G6PD acts as an important mediator of cellular defenses against ferroptosis by regenerating GSH and glutathione peroxidase 4 (GPX4). G6PD-d may thereby hinder anti-ferroptotic mechanisms.
However, as previously mentioned, intravascular hemolysis in G6PD-d is rare. While, extravascular RBC destruction by the reticuloendothelial system predominately occurs in the spleen and liver, RBCs have been shown to infiltrate the vascular endothelium in early atherosclerotic plaques64. Here, they are rapidly oxidized, recognized by scavenger receptors, and then phagocytosed by macrophages and macrophage-like VSMCs, which induces foam cell formation and plaque progression65. Given the effects of G6PD-d on endothelial permeability and redox homeostasis, RBC infiltration into the arterial wall and transformation of RBCs into inflammatory oxRBCs are likely both augmented. The ensuing phagocytosis of RBCs may lead to iron overload within macrophages, which has been shown to cause progressive macrophage dysfunction, inflammatory M1 polarization, increased ferroptosis and exacerbated atherosclerosis66,67,68,69,70,71. Free heme has also been shown to promote inflammatory gene transcription in macrophages via TLR4 and NLRP3 inflammasome signaling, which have both been implicated in worsened atherosclerosis72,73,74,75 (Fig. 3b).
Ferroptosis is a form of regulated cell death initiated by iron- or copper-mediated lipid peroxidation. Although thoroughly reviewed in other publications, there is mounting evidence in recent years linking ferroptosis in VECs, VSMCs and macrophages to worsened atherosclerosis62,76,77,78. Treatment of hyperlipemic mice with the ferroptosis inhibitor ferrostatin-1 (Fer-1) significantly decreases the development of atherosclerosis in ApoE-/- mice fed HFD for 12–16 weeks79. A corresponding role for ferroptosis in human ASCVD is also supported by a genetic analysis of human coronary artery plaques which demonstrated a significant positive association between ferroptosis-related gene expression and ASCVD status80,81.
Cells can stave off ferroptotic fate through the use of antioxidant pathways that largely utilize GSH, glutathione peroxidase 4 (GPX4), and perioredoxin-thioredoxin, which all ultimately rely on NADPH for regeneration. Imbalance in these regulatory antioxidant mechanisms and lipid peroxidation can eventually lead to ferroptosis82. In a murine model of renal cell carcinoma, upregulation of G6PD and subsequent increase in NADPH was found to inhibit ferroptosis whereas G6PD knockdown in human hepatocellular carcinoma cells increased positive regulators of ferroptosis83,84,85. While these studies focus on cancer cells, they reveal that G6PD is an important negative regulator of cell intrinsic causes of ferroptosis. (Fig. 3c) G6PD-d may therefore promote proatherogenic ferroptosis in a variety of cell types including VECs, VSMCs, macrophages, and other immune cells even in the absence of RBC destruction.
Immune system and G6PD-deficiency
ASCVD is a chronic inflammatory disease mediated by both the innate and adaptive arms of the immune system. Despite possibly evolving to mitigate the risk of malaria, the exact impact of G6PD-d on different immune cells is unclear. Accumulating evidence suggests that G6PD-d may lead to some degree of immune dysfunction and dysregulation13,14. In a retrospective cohort study of 7473 G6PD-d individuals, Israel et al. found increased rates of autoimmunity, allergy, infectious diseases, and other inflammatory diseases. These findings were accompanied by increased autoimmune antibody titers, IgG and IgA titers, and erythrocyte sedimentation rates as a marker of general inflammation13.
Immune cells are likely affected by G6PD-d in two major ways. First, G6PD-d limits the production of important substrates for biosynthesis, cell growth and proliferation. Ribose-5-phosphate is a precursor for ribonucleic acids required for cell growth, proliferation, and transcription. Likewise, NADPH is necessary for many reductive biosynthetic pathways including those generating fatty acids, nucleotides, and amino acids, all of which are also necessary for growth, survival, and proliferation12,86. (Fig. 4a) G6PD is commonly overexpressed in many myeloid and lymphoid malignancies leading to improved proliferation, but this effect can be abrogated by pharmacologic inhibition of G6PD87. Thus, G6PD-d may modulate immune functions by limiting rapid lymphocyte proliferation and differentiation that are key components of adaptive immunity.
a G6PD is important for the generation of NADPH and ribose-5-phosphate (R5P). These molecules support several reductive biosynthetic pathways including fatty acid, amino acid and nucleotide biosynthesis. In turn, these processes support cellular growth and proliferation. G6PD-d therefore may hinder growth and proliferation. b ROS are generated by NADPH-dependent and NADPH-independent processes. For example, NADPH oxidases (NOX) generate NADPH-dependent ROS while mitochondrial membrane proteins (i.e. complexes I and III), peroxisomal β-oxidation, and various other enzymes generate NADPH-independent ROS. Glutathione and thioredoxin represent two important antioxidant molecules that resolve accumulated ROS and rely on a functional G6PD enzyme for regeneration. c ROS are generated upon engagement of several immune cell receptors including toll-like receptors (TLR), B cell receptors (BCR), and T cell receptors (TCR). ROS from different sources can affect protein phosphatases, kinases and transcription factors, ultimately altering several different signaling pathways. Examples of such signaling pathways, proteins or protein complexes that have been shown to be influenced by ROS include NFκB, MAPK, PI3K and NLRP3. Thus, ROS have the potential to influence immune cell function through modification of signaling. The examples included are not exhaustive.
Another possible way in which G6PD-d may affect immune cells is through altering redox homeostasis. Without enough NADPH, cells lack sufficient GSH to aid in the neutralization of toxic ROS. Accumulation of oxidative stress secondary to G6PD-d has accordingly been shown to increase the propensity for cell death87,88. While NADPH is essential to regenerate most key antioxidant molecules, it also plays an important role in generating ROS through the action of NADPH oxidases (NOX). (Fig. 4a, b) NOX family enzymes are widely known for their role in oxidative burst utilized by neutrophils and macrophages to kill phagocytosed pathogens. Yet, accumulating evidence also highlights the ability of NOX family enzymes to modulate various immune cell effector functions through the production of ROS that alter signaling cascades and subsequent gene transcription89,90,91,92,93,94.
While the role of ROS as second messengers has been thoroughly reviewed in previous publications, we will broadly introduce the topic as ROS may have a significant role in the immune phenotypes observed in G6PD-d. Most evidence supports an activating and proinflammatory role for ROS in immune cells and nonimmune cells, including VEC and VSMC28,29,30,89,90,92,93. However, the outcomes of ROS-protein interactions are complex and dependent on several contextual factors including the concentration and type of ROS, the cell type, and the specific signaling pathways that are altered. Ligand-interactions with several immune cell surface receptors have been shown to induce controlled fluxes in ROS through the activation of NOX family enzymes as well as mitochondrial enzymes (complexes I and III of the electron transport chain). These receptors include, but are not limited to, the Toll-like receptor (TLR) family, the T cell receptor (TCR), and the B cell receptor (BCR). The ROS produced upon engagement of these receptors can then alter downstream signaling by oxidizing specific cysteine residues within a variety of intracellular proteins including protein kinases, protein phosphatases, adaptor proteins, calcium channels, and transcription factors. Examples of such signaling proteins that can be influenced directly or indirectly by ROS modifications include MAPK (ERK, JNK, p38), IKκB/NFκB, PI3K/AKT, IP3R/Ryanodine receptor, and the NLRP3 inflammasome90,92,93,94 (Fig. 4c).
ROS derived from NOX family enzymes are inherently dependent on NADPH produced by G6PD while mitochondrial ROS, for example, are largely NADPH-independent. Therefore, depletion of NADPH stores in G6PD-d has the potential to both limit the production of ROS by NOX family enzymes, while simultaneously expediting the accumulation of ROS from non-NADPH-dependent sources, such as the mitochondrial electron transport chain, peroxisomal β-oxidation, oxidative protein folding, and other cytoplasmic enzymes (xanthine oxidase, cyclooxygenase, lipoxygenase)95,96. (Fig. 4b) Whether these accumulated ROS can alter signal transduction has not been examined in the context of G6PD-d.
Throughout the following sections, we will propose the potential for two opposing effects of G6PD on immune cells. In the setting of acute stimulation, signaling may be dampened due to the lack of NADPH necessary for NOX function. The biosynthetic limitations imposed by G6PD-d may also limit proliferation, differentiation, and survival required for an effective immune response. Alternatively, ROS accumulation in response to ongoing physiologic and pathologic stressors may prime cells toward inflammation. These seemingly paradoxical effects may be one explanation for why patients with G6PD-d display increased susceptibility to infection indicative of an impaired immune response, while also displaying increased autoimmunity and inflammation indicative of an overactive immune system13. In the following sections, we will use this framework to examine emerging evidence of the impact of G6PD-d on three major ASCVD-mediating immune cells: macrophages, T cells and B cells.
Myeloid compartment
Monocytes/Macrophages and ASCVD
Classical monocyte-derived macrophages are important in the early steps of atherogenesis as they contribute significantly to the creation of an inflammatory microenvironment. Circulating monocytes are recruited to sites of inflammation by cell adhesion molecules and chemoattractants, which are known to be upregulated at baseline in G6PD-d28,29. Upon recruitment to developing plaques, monocytes encounter many inflammatory stimuli such as damage associated molecule patterns (DAMP), oxLDL, and IFNγ that trigger inflammatory cascades after engagement with toll-like receptors (TLR), scavenger receptors and cytokine receptors, respectively. Depending on the signals received in the plaque microenvironment, recruited monocytes can assume variety of macrophage identities including inflammatory macrophages, aortic intima-resident macrophages (MacAIR), and type I interferon-inducible cells. These subsets are all considered proatherogenic2. Monocyte-derived macrophages, inflammatory macrophages and TREM2+ macrophages can also form foam cells due to the intracellular accumulation of lipids. Inflammatory macrophages are the major contributor to necrotic core formation, yet TREM2+ macrophages have recently been shown to have low expression of inflammatory genes and a beneficial role in atherosclerosis97,98,99,100,101,102. In response to lipid accumulation and inflammatory signals, VSMCs can also differentiate into pro-inflammatory macrophage-like cells and foam cells that contribute to plaque growth103. Broadly speaking, inflammatory macrophages increase production of proinflammatory cytokines IL-6, TNFα, IL-1β, and type I interferons that create a harmful feedforward immune cell recruitment-activation cycle2,74,104,105. In contrast, some macrophages are considered anti-inflammatory as they are primarily responsible for efferocytosis of dead cells and debris to limit plaque formation. In the face of foam cell buildup and excessive cell death, efferocytes can become overwhelmed, leading to the loss of atheroprotective effector functions, cell death, and the release of pro-inflammatory DAMPs, which all augment the proatherogenic environment2. Failure of efferocytosis has been shown to worsen the development of atherosclerotic plaques106.
G6PD-Deficiency in Monocytes and Macrophages
Parsanathan et al. found that G6PD-d alone may worsen baseline inflammation in monocytes and macrophages. Interestingly, this effect is augmented by the presence of hyperglycemia, indicating that comorbid diabetes may enhance the proatherogenic phenotype of G6PD-d. In their study, human-derived monocytes were treated with either PMA, pharmacologic G6PD inhibitor 6-AN, G6PD-silencing siRNA, siRNA + hyperglycemia, or hyperglycemia alone and then inspected for changes in monocyte polarization without an immunogenic stimulus. While the G6PD-d monocytes displayed the phenotypic hallmarks of M2 polarization (CD11b, CD209 expression), these cells were functionally deficient in M2 atheroprotective TGFβ and had increased expression of proinflammatory M1 cytokines TNFα and MCP-1. NOX activity and ROS were notably increased in the G6PD-d groups as well. Altogether, these data support the hypothesis that G6PD-d drives ROS accumulation and may thereby amplify proinflammatory signaling or gene transcription in the absence of an acute stimulus30. While increased NOX activity may drive ROS production in the setting of sufficient NADPH stores, other NADPH-independent ROS may represent a more abundant alternative source of oxidative stress in this model of G6PD-d. (Fig. 5a) The source of ROS and the mechanism by which these ROS drive possibly drive this proinflammatory phenotype therefore warrant further investigation.
a G6PD-d monocytes accumulate ROS at baseline that paradoxically upregulate NOX. G6PD-d monocytes may be polarized to an M1-like phenotype characterized by the production of proinflammatory TNFɑ and MCP-1. Concurrently, reduction in M2 polarization may reduce anti-inflammatory TGFβ expression. This effect of G6PD-d would be proatherogenic though further studies are needed. b Plaque resident macrophages normally clear foam cells and debris from atherosclerotic plaques through efferocytosis, thereby preventing plaque growth and necrotic core formation. This process is inherently oxidative and relies on G6PD/PPP to maintain both redox homeostasis and continual efferocytosis. In G6PD-d, efferocytes may therefore be more prone to oxidative damage and death. Efferocyte death impairs clearance of apoptotic cells and debris while also increasing inflammation through the release of DAMPs. Further studies are needed to assess this effect in the setting of atherosclerosis.
As previously stated, G6PD-d promotes cell death by reducing the ability to cope with oxidative stress and to produce the macromolecules needed for proliferation and cellular repair12,87. When macrophages encounter efferocytic stimuli, glucose is preferentially shunted to the PPP to counter the oxidative stress caused by efferocytosis. In doing so, the PPP also supports continual efferocytosis. When G6PD is inhibited in murine cells both in vivo using 6-AN or knockdown, or in vitro using 6-AN or a novel selective small molecule inhibitor of G6PD (G6PDi-1), efferocytosis of apoptotic cells is significantly diminished107,108,109. Without a fully functioning PPP, intraplaque cellular death is likely increased and the ensuing atheroprotective process of efferocytosis may be dysfunctional. Additionally, when outpaced by excessive debris accumulation from intraplaque cell death, efferocytes die, leading to the release of inflammatory DAMPS and unhindered necrotic core formation2. Together, increased intraplaque cellular death and dysfunctional efferocytosis as a result of G6PD-d may accelerate plaque growth, necrotic core formation and the development of an inflammatory plaque microenvironment106,107 (Fig. 5b).
Sufficient G6PD activity appears to be crucial for inflammatory macrophage function as well. In vitro stimulation of human and murine macrophages and monocytes have demonstrated a proinflammatory effect of G6PD overexpression and a corresponding dampened response with G6PD-d110. G6PD overexpression has been shown to enhance p38 MAPK and NFκB signaling through the production of ROS by NOX. In doing so, G6PD increases the production of proatherogenic cytokines including IL-6, TNFα, IL-1β, and MCP-1 when stimulated. Likewise, siRNA knockdown and pharmacologic inhibition of G6PD reduce the observed proinflammatory phenotypes in vitro when stimulated110,111,112. Furthermore, PBMCs derived from G6PD-d donors and G6PD-knockdown human monocytes displayed reduced signaling through the NLRP3 inflammasome, p38 MAPK and c-Fos caused by decreased modulatory NADPH-dependent ROS. This reduced the production of pro-atherogenic IL-1β110.
The conflicting phenotypes in G6PD-d monocytes and macrophages highlight the difficulty in extrapolating in vitro data to living organisms. It is evident that there is a great deal of variability in phenotypes observed based on the presence or absence of stimulation, as well as in the type of macrophage examined. Thus, it is imperative that more in vivo studies focusing specifically on atherosclerosis are done to better understand how monocyte and macrophage biology is altered in the setting of G6PD-d.
Lymphoid compartment
T Cells in atherosclerosis
T cells are important adaptive immune regulators of atherosclerosis, demonstrating both atheroprotective and proatherogenic effects depending on the specific T cell subset. While the exact of roles of CD8 + T cells as well as many of the CD4 + T cell subsets (i.e. Th2, Th17, Th22, and Tfh cells) remain controversial, there is a propensity of evidence to support a clear proatherogenic role for Th1 cells and an atheroprotective role for Tregs. Th1 cells promote lesion development and plaque instability through the production of proinflammatory IFNγ and TNFα. Tregs limit plaque progression and promote plaque stabilization through the production of immunosuppressive cytokines TGFβ and IL-102,4. However, Tregs can also adopt an inflammatory effector-like phenotype and lose their suppressive capacity due to chronic inflammation, metabolic reprogramming, tissue-specific reprogramming, and possibly insufficient IL-2 signaling113,114. These so-called “exTregs” lose Foxp3 expression and are thought to be proatherogenic given the loss of their atheroprotective suppressor functions and the initiation of proinflammatory cytokine secretion2. In addition to Th1 cells and exTregs, the T follicular helper (Tfh) subset is also considered proatherogenic as these cells can direct humoral immunity toward a proinflammatory autoimmune phenotype through the production of IL-21. In contrast, the Th2 subset has a largely atheroprotective effect mediated by IL-5, which enhances the atheroprotective functions of B1 cells that will be discussed later, as well as IL-13, which promotes plaque stabilization. Th2 cells can produce IL-4 as well which has a controversial role in atherosclerosis. Th17 and Th22 cells, meanwhile, have been observed to display atheroprotective and proatherogenic effects and therefore warrant continued investigation. CD8 + T cell populations have been detected within human and murine atherosclerotic plaques. Like Th17 and Th22 cells, both atheroprotective and proatherogenic roles for CD8 + T cells have been documented. A population of regulatory CD8 + T cells may protect against plaque development by selectively killing immune cells that contribute to the overall inflammatory microenvironment. Conversely, another subset of CD8 + T cells can augment vascular inflammation through the production of IFNγ and TNF, and worsen plaque instability by killing VSMCs and VECs2,4.
G6PD-Deficiency in T cells
Based on what has been presented until this point, one may hypothesize how extrinsic signals from other G6PD-d cells might direct a T cell contribution to ASCVD. G6PD-d stimulates VECs to upregulate expression of chemoattractants and leukocyte adhesion molecules that could drive T cell recruitment to plaques28,29,115. T cells that have been recruited to atherosclerotic plaques likely experience increased cell death, which may further stress efferocytes and contribute to an inflammatory microenvironment87,88. Additionally, the increased M1 polarization observed by Parsanathan et al. has the potential to skew peripheral T cell differentiation toward a Th1 predominance through the secretion of M1-derived cytokines, such as IL-12 and IFNγ30.
Unlike the studies done by Parsanathan et al. on G6PD-d in monocytes and macrophages, there have been no studies to investigate whether G6PD-d results in a similar proinflammatory T cell phenotype in the absence of stimulation. However, like the literature on G6PD-d in monocytes and macrophages, it is evident that G6PD-d hinders the function of inflammatory T cells when stimulated. Ghergurovich et al. demonstrated that T cells increase flux through the PPP upon activation and that activated murine CD8+ and CD4 + T cells rely predominantly on G6PD for the production of NADPH. Treatment with a novel selective small molecule inhibitor of G6PD (G6PDi-1) prior to T cell restimulation consequently blocked the production of proatherogenic cytokines TNFɑ and IFNγ by CD8 + T cells as well as IL-2 and TNFɑ by CD4 + T cells. (Fig. 6a, b) Delaying G6PDi-1 treatment for 1 h post-stimulation allowed for substantial cytokine production, indicating that G6PD and the PPP likely play an important role in the early signaling events following stimulation. Similar to what is observed in monocytes and macrophages, G6PD-d may limit the production of NADPH-dependent ROS that act as second messengers to enhance NFAT, AP-1, and NFκB signaling89,116,117. G6PD-d may consequently hinder activation, differentiation, proliferation and the expansion of Th1 and CD8 + T cells during acute stimulation89,118,119. In contrast to the CD4+ and CD8 + T cell phenotypes observed, Ghergurovich et al. noted that the atheroprotective suppressor functions of CD4 + /CD25+ Treg cells were not affected in their model118. These in vitro results have been further validated in murine models of malaria infection and rheumatoid arthritis, where G6PD-d or inhibition leads to less severe proinflammatory responses to stimulation marked by reduced Th1 and Th17 differentiation, decreased proatherogenic cytokines IL-1β, IL-6, IL-12, and TNFɑ, and no impact on IL-10 and TGFβ (classically Treg-derived)120,121,122,123,124. However, these models are poor substitutes for in vivo models of atherosclerosis that involve different stimuli, immune populations, and timing.
G6PD-d has generally been shown to reduce cytokine production by T cells. The impact of this effect on atherosclerosis would be dependent on whether proatherogenic or atheroprotective T cell subsets, and their cytokine production, are affected more. a Th1 and Tfh cells are considered proatherogenic. Given a reduction in their cytokines would be atheroprotective, the CVD phenotype of G6PD-d would likely not be driven by changes in these cells. b Similar to Th1 and Tfh cells, CD8 + T cell expression of TNFɑ and IFNγ is reduced by G6PD inhibition and would be atheroprotective. G6PD activity also supports granzyme B production. Reduction in granzyme B-mediated destruction of VSMCs and VECs would be atheroprotective, but impaired killing of inflammatory T cells and APCs may represent a potential proatherogenic mechanism of G6PD-d. c The roles of Th2, Th17 and Th22 cells in atherosclerosis are unclear with both atheroprotective and proatherogenic functions reported. Like Th1 and CD8 + T cells, G6PD-d may limit cytokine production by these subsets but the impact of atherosclerosis depends on whether the proatherogenic or atheroprotective functions are relatively more impaired. d Tregs produce atheroprotective IL-10 and TGFβ. However, G6PD-d increases chronic inflammation and reduces Th1 production of IL-2. Both of these conditions may result in proatherogenic exTreg formation.
While suppression of the proatherogenic Th1 subset may confer some protection from atherosclerosis in G6PD-d, it is necessary to determine whether the other T cell subsets, particularly the atheroprotective subsets, are similarly affected. The overall effect of cytotoxic CD8 + T cells depends on which cells they target4. Given G6PD is an important metabolic immune checkpoint that supports granzyme B expression in CD8 + T cells, their cytotoxic function may be impaired125. (Fig. 6b) While this would impair killing of VSMC and VEC, which could help prevent ASCVD progression, it would similarly prevent CD8 + T cells from suppressing inflammatory CD4 + T cells, APCs that promote immune activation, and plasma cells that produce inflammatory IgG2,4. (Fig. 6b) Meanwhile, the atheroprotective effect of Th2 cells depends on production of IL-5 and IL-13. Given these cells are also CD4 + T cells like those studied by Ghergurovich et al., it is possible that production of these cytokines is impaired like their Th1 counterparts. (Fig. 6c) Although Tregs were noted to be unaffected by G6PD-d during in vitro and in vivo experiments, these studies did not examine the presence or function of exTregs. Ghergurovich et al. noted that IL-2 production was reduced by G6PD inhibition and Israel et al. found significantly increased markers of inflammation in human subjects with G6PD-d. These environmental signals, along with possible metabolic reprogramming secondary to G6PD-d, could be enough to promote proatherogenic exTreg differentiation. (Fig. 6d) Overall, further investigation in an in vivo model of atherosclerosis will be crucial to understanding how G6PD-d disrupts the balance of these proatherogenic and atheroprotective T cell effects.
B cells in ASCVD
B cells are highly dynamic regulators of atherosclerosis, influencing plaque development and progression through the secretion of cytokines and antibodies. While the role of B cells in ASCVD has been reviewed thoroughly elsewhere, we will briefly summarize with a focus on antibody producing functions2,3,126. There are two main classes of B cells in mice–– B1 and B2. B1 cells arise from the fetal liver and adult bone marrow but reside and self-renew through homeostatic proliferation primarily in the peritoneal and pleural cavities. Upon stimulation via TLR or BCR signaling, B1 cells produce natural IgM in a T cell-independent manner. Oxidation specific epitopes (OSE) present on the surface of oxLDL are major targets for natural IgM (IgMOSE). These IgMOSE bind to and facilitate the clearance of oxLDL from plaques while also blocking the OSEs from acting as inflammatory DAMPs. As a result, IgMOSE have been shown to reduce atherosclerosis in mice and to be inversely associated with coronary artery disease and coronary events in humans127,128,129,130,131. B1 cells can be further subdivided into B1a (CD5+) and B1b (CD5-) cells. B1a cells express germline-encoded natural IgM with little to no affinity maturation, resulting in IgM that is broadly specific and low-affinity for many self-antigens (including oxLDL). B1b cells are able to undergo somatic hypermutation and therefore have a more expanded IgM repertoire that is primarily secreted following stimulation through a TLR or BCR3,126.
B2 cells are divided into marginal zone (MZB) and follicular (FoB) B cells. Like B1 cells, MZB cells produce T cell-independent natural IgMOSE that has undergone somatic hypermutation and affinity maturation3,126. While there is debate about whether a human correlate to B1 cells exists, our group recently described a CD27+IgM+CD24hi MZ-like B cell population in the peripheral circulation of humans that produces IgMOSE and correlates with atheroprotection132. In contrast to B1 and MZB cells, FoB cells are largely considered pro-atherogenic and produce high affinity IgGOSE. In contrast to the IgMOSE produced by B1 and MZB cells, the role of IgGOSE in atherosclerosis is less clear and may depend on the subtype of IgG. Some IgG subtypes form immune complexes with oxLDL that stimulate an inflammatory macrophage response and thereby worsen plaque development, while others may be atheroprotective3,126,133 (Fig. 7a).
a B cell subsets can be atheroprotective (B1a, B1b, MZB) or proatherogenic (FoB). Atheroprotective B cells produce IgM to oxidation specific epitopes (IgMOSE). Proatherogenic FoB cells produce IgG (IgGOSE) which can form immune complexes with oxLDL that promote inflammatory macrophage responses or may sequester and clear oxLDL, depending on the IgG subclass and its receptor. In G6PD-d, plasma cell differentiation, IgMOSE and IgGOSE may all be reduced due to b impaired ROS production following BCR/TLR engagement and c impaired redox homeostasis inhibiting proper oxidative protein folding. d ROS may also inhibit BLIMP-1 expression, which is critical in promoting plasma differentiation and IgM production. e B1a and B1b cells require fatty acids (FA) to sustain self-renewal and survival. They acquire a significant proportion of FA through autophagy. However, in G6PD-d, deficient NADPH leads to reduced FA synthesis and accumulation of ROS due to insufficient GSH. ROS accumulation may then impair autophagy. Together, these factors may contribute to reduced self-renewal of important atheroprotective B cells. The increased ROS may also inhibit B1 migration to sites that support IgMOSE production (i.e. spleen). Lastly, ROS may disproportionately promote cell death or ferroptosis in atheroprotective B1 and MZB cells given these subsets are uniquely susceptible to lipid peroxidation compared to proatherogenic FoB cells.
G6PD-Deficiency and PPP in B cells
While limited in generalizability to B cells, one study of antibody production in industrial fed-batch hamster ovarian carcinoma cells demonstrated peak flux through the PPP during the late exponential phase of IgG production and sustained PPP activity through the stationary and decline phases. Peak production was also accompanied by increased oxidative metabolism, increased NADPH consumption, and decreased GSH. This indicates that the PPP has an important role in antibody production, likely by maintaining redox homeostasis during a time of heightened oxidative metabolism. As noted by the authors, GSH can also directly participate in the formation of disulfide bonds necessary for antibody production and NADPH is necessary for continual lipid rearrangement required for Ig secretion134,135. In a study by Brookens et al., when the PPP was inhibited in murine splenic B cells by DHEA or 6-AN, production of IgM (only by 6-AN) and IgG1 (by DHEA and 6-AN) following LPS stimulation decreased in a dose-dependent manner. While ROS were also increased by day 2 post-stimulation, restoration of PPP activity and redox homeostasis using galactose by 48 h did not restore antibody production so long as glycolytic flux was suboptimal. While this could indicate that flux through the PPP is necessary to compensate for oxidative processes downstream of glycolysis, it is also possible that optimal PPP flux is needed during the initial steps of activation. CD138+ plasma cell differentiation was also increased by PPP activity but was inhibited, along with proliferation, during DHEA or 6-AN treatment136. Together, these data indicate that antibody production and plasma cell differentiation are inversely related to G6PD activity. How this might affect the risk of ASCVD is dependent on whether atheroprotective IgM production or proatherogenic IgG production is more significantly affected. Unfortunately, no data is available to answer this question in the context of hyperlipidemia (Fig. 7a–c).
As with T cell and myeloid functions, ROS play a significant role in antibody production by regulating BCR (and likely TLR) signaling and transcriptional control. Studies by Feng et al. and Wheeler et al. demonstrated that following BCR engagement, NOX family enzymes are transiently activated to create two controlled peaks in ROS. The first, mediated by NOX2, may negatively regulate T cell-dependent antibody production. The second peak, mediated at least in part by NOX1, NOX3 and mitochondrial enzymes, results in prolonged signaling through PI3K, NF-κB, and AKT. As was observed previously with pharmacologic G6PD inhibition, these ROS impaired plasma cell differentiation and antibody production in splenic B cells. This effect was reversed by deletion of NOX1-4, NOX3 alone, or treatment with the ROS scavenger N-acetyl cysteine (NAC). (Fig. 7b) In contrast to the previous study that showed reduced proliferation with high ROS secondary to G6PD inhibition, proliferation in this study was increased when ROS were increased in the setting of normal G6PD activity. This further highlights the important biosynthetic role of G6PD in cell survival and proliferation87,93,94. Repression of Blimp-1 may represent one potential explanation for the effect of G6PD-inhibition on differentiation and antibody production. In the setting of high ROS, as is seen with G6PD-inhibition, heme production has been shown to be impaired137. Heme is an important inhibitor of BACH2, which represses transcription of Blimp-1138. By impairing heme production, high ROS can increase BACH2-dependent Blimp-1 repression. The consequent low production of BLIMP-1 decreases plasma cell differentiation and antibody production, which mirrors the effect of increased ROS on splenic B cells observed both with and without G6PD inhibition137,138,139,140 (Fig. 7d).
Peritoneal B1 cells are metabolically distinct from splenic FoB cells, relying on high levels of TCA, glycolysis and oxPPP to sustain homeostatic self-renewal at least in part through significant lipid metabolism141. MZB cells also rely disproportionately on lipid metabolism when compared to FoB cells, suggesting that lipid metabolism is important for natural antibody production142. In B1a cells, homeostatic autophagy supports fatty acid synthesis and impairment in either leads also to B1a cell death141. Three potential implications for G6PD-d in atheroprotective B cells arise from this dependence on fatty acid synthesis. First, G6PD-derived NADPH is a necessary cofactor for fatty acid synthesis and deficiency in NADPH may therefore lead to impairment and cell death. Second, autophagy is inhibited by ROS143. In the setting of chronic ROS accumulation, B1 cells may experience impaired autophagy leading to inhibited homeostatic proliferation or death. Third, given their reliance on lipid metabolism, B1 and MZB cells are extremely susceptible to lipid peroxidation and subsequent ferroptosis secondary to ROS accumulation142. G6PD-d may therefore disproportionately affect B1 and MZB cells, giving rise to deficits in IgMOSE while not having as much of an impact on proatherogenic FoB cells. Finally, B1 cells need to migrate to niches that support antibody production in order to increase secretion of IgMOSE. G6PD inhibition has been shown to impair cellular migration through ROS-dependent inhibition of protein phosphatases involved in cytoskeletal rearrangement, suggesting that chronic ROS accumulation in G6PD-d may inhibit B1 migration necessary for IgMOSE production87,144,145 (Fig. 7e).
Altogether, both atheroprotective and proatherogenic antibody production may be impaired by G6PD-d. However, there may be a disproportionate impact on atheroprotective IgM production by B1 and MZB cells due to the unique metabolic needs and functions of these subsets. Again, further investigation using in vivo models of atherosclerosis will be essential to understanding how G6PD-d in B cells affects ASCVD risk.
Concluding remarks
Although epidemiological evidence suggests that G6PD-d worsens atherosclerosis, the mechanisms by which this occurs are unknown. G6PD-d has the powerful potential to yield both atheroprotective and proatherogenic outcomes through its effects on cell survival, proliferation, activation, and cellular signaling in RBCs, VECs, VSMCs and different immune cell subsets. A careful examination of the impact of G6PD-d on each of the key players in atherogenesis is overdue and likely on the horizon. Furthermore, understanding the effect of varying levels of G6PD activity on each of the key immune players in ASCVD will be crucial to developing targeted G6PD-immunomodulatory therapies and predicting ASCVD risk in patients. Many of the experiments reviewed here rely on pharmacologic G6PD inhibition or G6PD siRNA knockdown. While pharmacologic inhibition may not induce enough inhibition, G6PD knockdown may go too far, and neither recapitulates the protein instability that is characteristic of human G6PD-d. Thus, more models using humanized mice and donated patient samples will be critical in future experiments. This review is not comprehensive as we only focused on a subset of the immune cells known to modify ASCVD and were only able to broadly cover ROS modification of signaling as it likely pertains to G6PD-d. In reality, G6PD-d, immunity, ROS, ferroptosis, and ASCVD are all complicated topics alone and even more so when exploring the connections among them. We hope this review provides a starting point on which future publications can build and deepen our understanding of these important topics.
Data availability
No datasets were generated or analysed during the current study.
References
The top 10 causes of death. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
Roy, P., Orecchioni, M. & Ley, K. How the immune system shapes atherosclerosis: roles of innate and adaptive immunity. Nat. Rev. Immunol. 22, 251–265 (2022).
Sage, A. P., Tsiantoulas, D., Binder, C. J. & Mallat, Z. The role of B cells in atherosclerosis | Nature Reviews Cardiology. Nat. Rev. Cardiol. 16, 180–196 (2019).
Saigusa, R., Winkels, H. & Ley, K. T cell subsets and functions in atherosclerosis. Nat. Rev. Cardiol. 17, 387–401 (2020).
Biros, E., Karan, M. & Golledge, J. Genetic Variation and Atherosclerosis. Curr. Genomics 9, 29–42 (2008).
Pattarabanjird, T. et al. A Machine Learning Model Utilizing a Novel SNP Shows Enhanced Prediction of Coronary Artery Disease Severity. Genes 11, 1446 (2020).
Wajcman, H. & Galactéros, F. Glucose 6-phosphate dehydrogenase deficiency: a protection against malaria and a risk for hemolytic accidents. C. R. Biol. 327, 711–720 (2004).
D’Alessandro, A. et al. Hematologic and systemic metabolic alterations due to Mediterranean class II G6PD deficiency in mice. JCI Insight 6, e147056 (2021).
Vera Kazakova & Patan Gultawatvichai. Glucose-6-Phosphate Dehydrogenase Deficiency - ClinicalKey. In Ferri’s Clincal Advisor 2025 473e30-473e32 (Elsevier, 2024).
Anna L. Peters & Cornelis J. F. Van Noorden. Glucose-6-phosphate Dehydrogenase Deficiency and Malaria: Cytochemical Detection of Heterozygous G6PD Deficiency in Women. 57, 1003–1011 (2009).
Global Malaria Programme (GMP) & Malaria Policy Advisory Group. Meeting report of the technical consultation to review the classification of glucose-6-phosphate dehydrogenase (G6PD).
TeSlaa, T., Ralser, M., Fan, J. & Rabinowitz, J. D. The pentose phosphate pathway in health and disease. Nat. Metab. 5, 1275–1289 (2023).
Israel, A. et al. Glucose-6-phosphate dehydrogenase deficiency and long-term risk of immune-related disorders. Front. Immunol. 14, 1232560 (2023).
Elsea, S. H. et al. Association of Glucose-6-Phosphate Dehydrogenase Deficiency With Outcomes in US Veterans With COVID-19. JAMA Netw. Open 6, e235626 (2023).
Long, W. K., Wilson, S. W. & Frenkel, E. P. Associations between red cell glucose-6-phosphate dehydrogenase variants and vascular diseases. Am. J. Hum. Genet. 19, 35–53 (1967).
Cocco, P. et al. Mortality in a Cohort of Men Expressing the Glucose-6-Phosphate Dehydrogenase Deficiency. Blood 91, 706–709 (1998).
Meloni, L. et al. Glucose-6-phosphate dehydrogenase deficiency protects against coronary heart disease. J. Inherit. Metab. Dis. 31, 412 (2008).
Thomas, J. E. et al. Glucose-6-Phosphate Dehydrogenase Deficiency is Associated with Cardiovascular Disease in U.S. Military Centers. Tex. Heart Inst. J. 45, 144–150 (2018).
Pes, G. M., Parodi, G. & Dore, M. P. Glucose-6-phosphate dehydrogenase deficiency and risk of cardiovascular disease: A propensity score-matched study. Atherosclerosis 282, 148–153 (2019).
Li, J. et al. Glucose-6-phosphate dehydrogenase deficiency and intracranial atherosclerotic stenosis in stroke patients. Eur. J. Neurol. 29, 2683–2689 (2022).
Ou, Z. ilin et al. Glucose-6-phosphate dehydrogenase deficiency and stroke outcomes | Neurology. Neurology 95, e1471–e1478 (2020).
Dore, M. P., Portoghese, M. & Pes, G. M. The Elderly with Glucose-6-Phosphate Dehydrogenase Deficiency are More Susceptible to Cardiovascular Disease. J. Atheroscler. Thromb. 28, 604–610 (2021).
Dore, M. P., Parodi, G., Portoghese, M. & Pes, G. M. The Controversial Role of Glucose-6-Phosphate Dehydrogenase Deficiency on Cardiovascular Disease: A Narrative Review. Oxid. Med. Cell. Longev. 2021, 5529256 (2021).
Zhao, J. et al. The association between glucose-6-phosphate dehydrogenase deficiency and abnormal blood pressure among prepregnant reproductive-age Chinese females. Hypertens. Res. 42, 75–84 (2019).
Lai, Y. K., Lai, N. M. & Lee, S. W. H. Glucose-6-phosphate dehydrogenase deficiency and risk of diabetes: a systematic review and meta-analysis. Ann. Hematol. 96, 839–845 (2017).
Cappai, G., Songini, M., Doria, A., Cavallerano, J. D. & Lorenzi, M. Increased prevalence of proliferative retinopathy in patients with type 1 diabetes who are deficient in glucose-6-phosphate dehydrogenase. Diabetologia 54, 1539–1542 (2011).
Israel, A. et al. Type 2 Diabetes in Patients with G6PD Deficiency. N. Engl. J. Med. 391, 568–569 (2024).
Parsanathan, R. & Jain, S. K. Glucose-6-Phosphate Dehydrogenase Deficiency Activates Endothelial Cell and Leukocyte Adhesion Mediated via the TGFβ/NADPH Oxidases/ROS Signaling Pathway. Int. J. Mol. Sci. 21, 7474 (2020).
Parsanathan, R. & Jain, S. K. Glucose-6-phosphate dehydrogenase deficiency increases cell adhesion molecules and activates human monocyte-endothelial cell adhesion: Protective role of l-cysteine. Arch. Biochem. Biophys. 663, 11–21 (2019).
Parsanathan, R. & Jain, S. K. G6PD deficiency shifts polarization of monocytes/macrophages towards a proinflammatory and profibrotic phenotype. Cell. Mol. Immunol. 18, 770–772 (2021).
Gauthier, T. W., Scalia, R., Murohara, T., Guo, J. & Lefer, A. M. Nitric Oxide Protects Against Leukocyte-Endothelium Interactions in the Early Stages of Hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 15, 1652–1659 (1995).
Aguilar, G., Koning, T., Ehrenfeld, P. & Sánchez, F. A. Role of NO and S-nitrosylation in the Expression of Endothelial Adhesion Proteins That Regulate Leukocyte and Tumor Cell Adhesion. Front. Physiol. 11, 595526 (2020).
Zhao, Z. et al. S-nitrosylation of ARH is required for LDL uptake by the LDL receptor. J. Lipid Res. 54, 1550–1559 (2013).
Fukumura, D. et al. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc. Natl. Acad. Sci. 98, 2604–2609 (2001).
Leopold, J. A., Cap, A., Scribner, A. W., Stanton, R. C. & Loscalzo, J. Glucose-6-phosphate dehydrogenase deficiency promotes endothelial oxidant stress and decreases endothelial nitric oxide bioavailability. FASEB J. 15, 1771–1773 (2001).
Nielsen, L. B. Transfer of low density lipoprotein into the arterial wall and risk of atherosclerosis. Atherosclerosis 123, 1–15 (1996).
Varghese, M. V., James, J., Rafikova, O. & Rafikov, R. Glucose-6-phosphate dehydrogenase deficiency contributes to metabolic abnormality and pulmonary hypertension. Am. J. Physiol. -Lung Cell. Mol. Physiol. 320, L508–L521 (2021).
Signoretti, C. et al. G6pdN126D Variant Increases the Risk of Developing VEGFR (Vascular Endothelial Growth Factor Receptor) Blocker-Induced Pulmonary Vascular Disease. J. Am. Heart Assoc. 13, e035174 (2024).
Matsumura, S. et al. Mediterranean G6PD variant rats are protected from Angiotensin II-induced hypertension and kidney damage, but not from inflammation and arterial stiffness. Vasc. Pharmacol. 145, 107002 (2022).
Lehners, M., Dobrowinski, H., Feil, S. & Feil, R. cGMP Signaling and Vascular Smooth Muscle Cell Plasticity. J. Cardiovasc. Dev. Dis. 5, 20 (2018).
Melichar, V. O. et al. Reduced cGMP signaling associated with neointimal proliferation and vascular dysfunction in late-stage atherosclerosis. Proc. Natl. Acad. Sci. Usa. 101, 16671–16676 (2004).
Malek, A. M., Alper, S. L. & Izumo, S. Hemodynamic Shear Stress and Its Role in Atherosclerosis. JAMA 282, 2035–2042 (1999).
Chappell, D. C., Varner, S. E., Nerem, R. M., Medford, R. M. & Alexander, R. W. Oscillatory Shear Stress Stimulates Adhesion Molecule Expression in Cultured Human Endothelium. Circ. Res. 82, 532–539 (1998).
Jo, H., Dull, R. O., Hollis, T. M. & Tarbell, J. M. Endothelial albumin permeability is shear dependent, time dependent, and reversible. Am. J. Physiol. -Heart Circ. Physiol. 260, H1992–H1996 (1991).
Kitagawa, A. et al. CRISPR-Mediated SNP Modeling in Rats Reveals Insight into Reduced Cardiovascular Risk Associated with Mediterranean G6PD Variant. Hypertens. Dallas Tex. 1979 76, 523–532 (2020).
Li, Y. & Pagano, P. J. Does the Mediterranean G6PD S188F Polymorphism Confer Vascular Protection? A Novel Rat Model Offering CRISPr Insight Into High Fat-Induced Vascular Dysfunction & Hypertension. Hypertens. Dallas Tex. 1979 76, 314–315 (2020).
Karadsheh, N. S., Quttaineh, N. A., Karadsheh, S. N. & El-Khateeb, M. Effect of combined G6PD deficiency and diabetes on protein oxidation and lipid peroxidation. BMC Endocr. Disord. 21, 246 (2021).
Mallat, Z. et al. Protective Role of Interleukin-10 in Atherosclerosis. Circ. Res. 85, e17-24 (1999).
Mordmüller, B., Turrini, F., Long, H., Kremsner, P. G. & Arese, P. Neutrophils and monocytes from subjects with the Mediterranean G6PD variant: effect of Plasmodium falciparum hemozoin on G6PD activity, oxidative burst and cytokine production. Eur. Cytokine Netw. 9, 239–245 (1998).
Liese, A. M., Siddiqi, M. Q., Siegel, J. H., Deitch, E. A. & Spolarics, Z. Attenuated Monocyte Il-10 Production in Glucose-6-Phosphate Dehydrogenase-Deficient Trauma Patients. Shock 18, 18 (2002).
Spolarics, Z. et al. Increased incidence of sepsis and altered monocyte functions in severely injured type A− glucose-6-phosphate dehydrogenase-deficient African American trauma patients. Crit. Care Med. 29, 728 (2001).
Liao, S.-L., Lai, S.-H., Tsai, M.-H. & Weng, Y.-H. Cytokine Responses of TNF-α, IL-6, and IL-10 in G6PD-Deficient Infants. Pediatr. Hematol. Oncol. 31, 87–94 (2014).
Wilmanski, J., Siddiqi, M., Deitch, E. A. & Spolarics, Z. Augmented IL-10 production and redox-dependent signaling pathways in glucose-6-phosphate dehydrogenase-deficient mouse peritoneal macrophages. J. Leukoc. Biol. 78, 85–94 (2005).
Wilmanski, J., Villanueva, E., Deitch, E. A. & Spolarics, Z. Glucose-6-phosphate dehydrogenase deficiency and the inflammatory response to endotoxin and polymicrobial sepsis*. Crit. Care Med. 35, 510 (2007).
Upperman, J. S. et al. Dominance of High-Producing Interleukin 6 And Low-Producing Interleukin 10 And Interferon γ Alleles In Glucose-6-Phosphate Dehydrogenase- Deficient Trauma Patients. Shock 23, 197 (2005).
Phillips, J. & Henderson, A. C. Hemolytic Anemia: Evaluation and Differential Diagnosis. Am. Fam. Physician 98, 354–361 (2018).
Luzzatto, L., Ally, M. & Notaro, R. Glucose-6-phosphate dehydrogenase deficiency. Blood 136, 1225–1240 (2020).
Abdelhalim, M. & Moussa, S. Biochemical Changes of Hemoglobin and Osmotic Fragility of Red Blood Cells in High Fat Diet Rabbits. Pak. J. Biol. Sci. PJBS 13, 73–77 (2010).
Kumar, S. Study of oxidative stress in Hypercholesterolemia. International Journal of Contemporary Medical Research. Int. J. Contemp. Med. Res. 4, 1204–1207 (2017).
Donadee, C. et al. Nitric Oxide Scavenging by Red Cell Microparticles and Cell Free Hemoglobin as a Mechanism for the Red Cell Storage Lesion. Circulation 124, 465–476 (2011).
Roncone, R., Barbieri, M., Monzani, E. & Casella, L. Reactive nitrogen species generated by heme proteins: Mechanism of formation and targets. Coord. Chem. Rev. 250, 1286–1293 (2006).
Vinchi, F. et al. Atherosclerosis is aggravated by iron overload and ameliorated by dietary and pharmacological iron restriction. Eur. Heart J. 41, 2681–2695 (2020).
Horwitz, L. D. & Rosenthal, E. A. Iron-mediated cardiovascular injury. Vasc. Med. 4, 93–99 (1999).
Delbosc, S. et al. Erythrocyte Efferocytosis by the Arterial Wall Promotes Oxidation in Early-Stage Atheroma in Humans. Front. Cardiovasc. Med. 4, 43 (2017).
Schrijvers, D., Demeyer, G., Herman, A. & Martinet, W. Phagocytosis in atherosclerosis: Molecular mechanisms and implications for plaque progression and stability. Cardiovasc. Res. 73, 470–480 (2007).
Dufrusine, B. et al. Iron-Dependent Trafficking of 5-Lipoxygenase and Impact on Human Macrophage Activation. Front. Immunol. 10, 1347 (2019).
Handa, P. et al. Iron alters macrophage polarization status and leads to steatohepatitis and fibrogenesis. J. Leukoc. Biol. 105, 1015–1026 (2019).
Tolosano, E., Fagoonee, S., Morello, N., Vinchi, F. & Fiorito, V. Heme Scavenging and the Other Facets of Hemopexin. Antioxid. Redox Signal. 12, 305–320 (2010).
Malhotra, R. et al. Hepcidin Deficiency Protects Against Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 39, 178–187 (2019).
Cai, J. et al. Iron accumulation in macrophages promotes the formation of foam cells and development of atherosclerosis. Cell Biosci. 10, 137 (2020).
Yu, W. et al. High Level of Uric Acid Promotes Atherosclerosis by Targeting NRF2-Mediated Autophagy Dysfunction and Ferroptosis. Oxid. Med. Cell. Longev. 2022, 9304383 (2022).
Singh, R. K. et al. TLR4 (Toll-Like Receptor 4)-Dependent Signaling Drives Extracellular Catabolism of LDL (Low-Density Lipoprotein) Aggregates. Arterioscler. Thromb. Vasc. Biol. 40, 86–102 (2020).
Dutra, F. F. et al. Hemolysis-induced lethality involves inflammasome activation by heme. Proc. Natl. Acad. Sci. USA. 111, E4110–E4118 (2014).
Grebe, A., Hoss, F. & Latz, E. NLRP3 Inflammasome and the IL-1 Pathway in Atherosclerosis. Circ. Res. 122, 1722–1740 (2018).
Figueiredo, R. T. et al. Characterization of Heme as Activator of Toll-like Receptor 4 *. J. Biol. Chem. 282, 20221–20229 (2007).
Bao, X. et al. Cigarette tar mediates macrophage ferroptosis in atherosclerosis through the hepcidin/FPN/SLC7A11 signaling pathway. Free Radic. Biol. Med. 201, 76–88 (2023).
Martinet, W., Coornaert, I., Puylaert, P. & De Meyer, G. R. Y. Macrophage Death as a Pharmacological Target in Atherosclerosis. Front. Pharmacol. 10, 306 (2019).
Xu, X. et al. The mechanisms of ferroptosis and its role in atherosclerosis. Biomed. Pharmacother. 171, 116112 (2024).
Bai, T., Li, M., Liu, Y., Qiao, Z. & Wang, Z. Inhibition of ferroptosis alleviates atherosclerosis through attenuating lipid peroxidation and endothelial dysfunction in mouse aortic endothelial cell. Free Radic. Biol. Med. 160, 92–102 (2020).
Liu, H., Xiang, C., Wang, Z. & Song, Y. Identification of Potential Ferroptosis-Related Biomarkers and Immune Infiltration in Human Coronary Artery Atherosclerosis. Int. J. Gen. Med. 15, 2979–2990 (2022).
Lu, C., Lan, Q., Song, Q. & Yu, X. Identification and validation of ferroptosis-related genes for diabetic retinopathy. Cell. Signal. 113, 110955 (2024).
Yan, H. et al. Ferroptosis: mechanisms and links with diseases. Signal Transduct. Target. Ther. 6, 1–16 (2021).
Cao, F., Luo, A. & Yang, C. G6PD inhibits ferroptosis in hepatocellular carcinoma by targeting cytochrome P450 oxidoreductase. Cell. Signal. 87, 110098 (2021).
Miess, H. et al. The glutathione redox system is essential to prevent ferroptosis caused by impaired lipid metabolism in clear cell renal cell carcinoma. Oncogene 37, 5435–5450 (2018).
Zheng, Q. et al. Deficiency of the X-inactivation escaping gene KDM5C in clear cell renal cell carcinoma promotes tumorigenicity by reprogramming glycogen metabolism and inhibiting ferroptosis. Theranostics 11, 8674–8691 (2021).
Chandel, N. S. NADPH—The Forgotten Reducing Equivalent. Cold Spring Harb. Perspect. Biol. 13, a040550 (2021).
Yang, H.-C. et al. The Redox Role of G6PD in Cell Growth, Cell Death, and Cancer. Cells 8, 1055 (2019).
Daneshmandi, S. et al. Blockade of 6-phosphogluconate dehydrogenase generates CD8+ effector T cells with enhanced anti-tumor function. Cell Rep. 34, 108831 (2021).
Peng, H.-Y. et al. Metabolic Reprogramming and Reactive Oxygen Species in T Cell Immunity. Front. Immunol. 12, 652687 (2021).
Kong, X., Thimmulappa, R., Kombairaju, P. & Biswal, S. NADPH Oxidase-Dependent Reactive Oxygen Species Mediate Amplified TLR4 Signaling and Sepsis-Induced Mortality in Nrf2-deficient Mice. J. Immunol. Baltim. Md 1950 185, 569–577 (2010).
Sauer, H., Wartenberg, M. & Hescheler, J. Reactive Oxygen Species as Intracellular Messengers During Cell Growth and Differentiation. Cell. Physiol. Biochem. 11, 173–186 (2001).
Williams, M. S. & Kwon, J. T Cell Receptor Stimulation, Reactive Oxygen Species, and Cell Signaling. Free Radic. Biol. Med. 37, 1144–1151 (2004).
Feng, Y.-Y. et al. Essential Role of NADPH Oxidase–Dependent Production of Reactive Oxygen Species in Maintenance of Sustained B Cell Receptor Signaling and B Cell Proliferation. J. Immunol. 202, 2546–2557 (2019).
Wheeler, M. L. & DeFranco, A. L. Prolonged Production of Reactive Oxygen Species in Response to B Cell Receptor Stimulation Promotes B Cell Activation and Proliferation. J. Immunol. 189, 4405–4416 (2012).
de Almeida, A. J. P. O. et al. ROS: Basic Concepts, Sources, Cellular Signaling, and its Implications in Aging Pathways. Oxid. Med. Cell. Longev. 2022, 1225578 (2022).
Shergalis, A. G., Hu, S., Bankhead, A. & Neamati, N. Role of the ERO1-PDI interaction in oxidative protein folding and disease. Pharmacol. Ther. 210, 107525 (2020).
Gui, Y., Zheng, H. & Cao, R. Y. Foam Cells in Atherosclerosis: Novel Insights Into Its Origins, Consequences, and Molecular Mechanisms. Front. Cardiovasc. Med. 9, (2022).
Zernecke, A. et al. Meta-Analysis of Leukocyte Diversity in Atherosclerotic Mouse Aortas. Circ. Res. https://doi.org/10.1161/CIRCRESAHA.120.316903 (2020).
Cochain, C., Saliba, A.-E. & Zernecke, A. Letter by Cochain et al Regarding Article, “Transcriptome Analysis Reveals Nonfoamy Rather Than Foamy Plaque Macrophages Are Proinflammatory in Atherosclerotic Murine Models”. Circ. Res. 123, 1127–1142 (2018).
Endo-Umeda, K. et al. Myeloid LXR (Liver X Receptor) Deficiency Induces Inflammatory Gene Expression in Foamy Macrophages and Accelerates Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 42, 719–731 (2022).
Kim, K. et al. Transcriptome Analysis Reveals Nonfoamy Rather Than Foamy Plaque Macrophages Are Proinflammatory in Atherosclerotic Murine Models. Circ. Res. 123, 1127–1142 (2018).
Piollet, M. et al. TREM2 protects from atherosclerosis by limiting necrotic core formation. Nat. Cardiovasc. Res. 3, 269–282 (2024).
Bennett, M. R., Sinha, S. & Owens, G. K. Vascular smooth muscle cells in atherosclerosis. Circ. Res. 118, 692–702 (2016).
Kirii, H. et al. Lack of Interleukin-1β Decreases the Severity of Atherosclerosis in ApoE-Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 23, 656–660 (2003).
Ridker, P. M. et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Kojima, Y., Weissman, I. L. & Leeper, N. J. The Role of Efferocytosis in Atherosclerosis. Circulation 135, 476–489 (2017).
Tsai, T.-L. et al. Multiomics reveal the central role of pentose phosphate pathway in resident thymic macrophages to cope with efferocytosis-associated stress. Cell Rep. 40, 111065 (2022).
Wang, Y.-T. et al. Metabolic adaptation supports enhanced macrophage efferocytosis in limited-oxygen environments. Cell Metab. 35, 316–331.e6 (2023).
He, D. et al. Pentose Phosphate Pathway Regulates Tolerogenic Apoptotic Cell Clearance and Immune Tolerance. Front. Immunol. 12, 797091 (2022).
Yen, W.-C. et al. Impaired inflammasome activation and bacterial clearance in G6PD deficiency due to defective NOX/p38 MAPK/AP-1 redox signaling. Redox Biol. 28, 101363 (2020).
Ham, M. et al. Macrophage Glucose-6-Phosphate Dehydrogenase Stimulates Proinflammatory Responses with Oxidative Stress. Mol. Cell. Biol. 33, 2425–2435 (2013).
Park, J. et al. Increase in Glucose-6-Phosphate Dehydrogenase in Adipocytes Stimulates Oxidative Stress and Inflammatory Signals. Diabetes 55, 2939–2949 (2006).
Tang, Q. et al. Central role of a defective interleukin-2 production in triggering islet autoimmune destruction. Immunity 28, 687–697 (2008).
Freuchet, A. et al. Identification of human exTreg cells as CD16+CD56+ cytotoxic CD4+ T cells. Nat. Immunol. 24, 1748–1761 (2023).
Parsanathan, R. & Jain, S. K. Glucose-6-phosphate dehydrogenase (G6PD) deficiency is linked with cardiovascular disease. Hypertens. Res. 43, 582–584 (2020).
Kamiński, M. M. et al. T cell Activation Is Driven by an ADP-Dependent Glucokinase Linking Enhanced Glycolysis with Mitochondrial Reactive Oxygen Species Generation. Cell Rep. 2, 1300–1315 (2012).
Sena, L. A. et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 38, 225–236 (2013).
Ghergurovich, J. M. et al. A small molecule G6PD inhibitor reveals immune dependence on pentose phosphate pathway. Nat. Chem. Biol. 16, 731–739 (2020).
Bai, A. et al. NADH oxidase-dependent CD39 expression by CD8+ T cells modulates interferon gamma responses via generation of adenosine. Nat. Commun. 6, 8819 (2015).
Yi, H. et al. G6pd-Deficient Mice Are Protected From Experimental Cerebral Malaria and Liver Injury by Suppressing Proinflammatory Response in the Early Stage of Plasmodium berghei Infection. Front. Immunol. 12, 719189 (2021).
Erlandsson, M. C. et al. Survivin promotes a glycolytic switch in CD4+ T cells by suppressing the transcription of PFKFB3 in rheumatoid arthritis. iScience25, 105526 (2022).
Tsokos, G. C. Metabolic control of arthritis: Switch pathways to treat. Sci. Transl. Med. 8, 331fs8–331fs8 (2016).
Yang, Z. et al. Restoring oxidant signaling suppresses proarthritogenic T cell effector functions in rheumatoid arthritis. Sci. Transl. Med. 8, 331ra38 (2016).
Yang, Z., Fujii, H., Mohan, S. V., Goronzy, J. J. & Weyand, C. M. Phosphofructokinase deficiency impairs ATP generation, autophagy, and redox balance in rheumatoid arthritis T cells. J. Exp. Med. 210, 2119–2134 (2013).
Lu, C. et al. G6PD functions as a metabolic checkpoint to regulate granzyme B expression in tumor-specific cytotoxic T lymphocytes. J. Immunother. Cancer 10, e003543 (2022).
Pattarabanjird, T., Li, C. & McNamara, C. B Cells in Atherosclerosis: Mechanisms and Potential Clinical Applications. JACC Basic Transl. Sci. 6, 546–563 (2021).
Rosenfeld, S. M. et al. B-1b Cells Secrete Atheroprotective IgM and Attenuate Atherosclerosis. Circ. Res. 117, e28–e39 (2015).
Que, X. et al. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature 558, 301–306 (2018).
Pattarabanjird, T. et al. B-1b Cells Possess Unique bHLH-Driven P62-Dependent Self-Renewal and Atheroprotection. Circ. Res. https://doi.org/10.1161/CIRCRESAHA.121.320436 (2022).
Tsimikas, S. et al. Relationship of IgG and IgM autoantibodies to oxidized low density lipoprotein with coronary artery disease and cardiovascular events. J. Lipid Res. 48, 425–433 (2007).
Fraley, A. E. et al. Relationship of Oxidized Phospholipids and Biomarkers of Oxidized Low-Density Lipoprotein With Cardiovascular Risk Factors, Inflammatory Biomarkers, and Effect of Statin Therapy in Patients With Acute Coronary Syndromes. J. Am. Coll. Cardiol. 53, 2186–2196 (2009).
Pattarabanjird, T. et al. Human circulating CD24hi marginal zone B cells produce IgM targeting atherogenic antigens and confer protection from vascular disease. Nat. Cardiovasc. Res. 2, 1003–1014 (2023).
Deroissart, J., Binder, C. J. & Porsch, F. Role of Antibodies and Their Specificities in Atherosclerotic Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 44, 2154–2168 (2024).
Templeton, N., Dean, J., Reddy, P. & Young, J. D. Peak antibody production is associated with increased oxidative metabolism in an industrially relevant fed-batch CHO cell culture. Biotechnol. Bioeng. 110, 2013–2024 (2013).
Cura, A. J. et al. Metabolic understanding of disulfide reduction during monoclonal antibody production. Appl. Microbiol. Biotechnol. 104, 9655–9669 (2020).
Brookens, S. K. et al. Plasma Cell Differentiation, Antibody Quality, and Initial Germinal Center B Cell Population Depend on Glucose Influx Rate. J. Immunol. 212, 43–56 (2023).
Jang, K.-J. et al. Mitochondrial function provides instructive signals for activation-induced B-cell fates. Nat. Commun. 6, 6750 (2015).
Watanabe-Matsui, M. et al. Heme regulates B-cell differentiation, antibody class switch, and heme oxygenase-1 expression in B cells as a ligand of Bach2. Blood 117, 5438–5448 (2011).
Shaffer, A. L. et al. Blimp-1 Orchestrates Plasma Cell Differentiation by Extinguishing the Mature B Cell Gene Expression Program. Immunity 17, 51–62 (2002).
Zhou, Y., Wu, H., Zhao, M., Chang, C. & Lu, Q. The Bach Family of Transcription Factors: A Comprehensive Review. Clin. Rev. Allergy Immunol. 50, 345–356 (2016).
Clarke, A. J., Riffelmacher, T., Braas, D., Cornall, R. J. & Simon, A. K. B1a B cells require autophagy for metabolic homeostasis and self-renewal. J. Exp. Med. 215, 399–413 (2018).
Muri, J., Thut, H., Bornkamm, G. W. & Kopf, M. B1 and Marginal Zone B Cells but Not Follicular B2 Cells Require Gpx4 to Prevent Lipid Peroxidation and Ferroptosis. Cell Rep. 29, 2731–2744.e4 (2019).
Chang, K.-C. et al. The interplay of autophagy and oxidative stress in the pathogenesis and therapy of retinal degenerative diseases. Cell Biosci. 12, 1 (2022).
Fang, Z. et al. Effects of G6PD activity inhibition on the viability, ROS generation and mechanical properties of cervical cancer cells. Biochim. Biophys. Acta BBA - Mol. Cell Res. 1863, 2245–2254 (2016).
Hurd, T. R., DeGennaro, M. & Lehmann, R. Redox regulation of cell migration and adhesion. Trends Cell Biol. 22, 107–115 (2012).
Acknowledgements
I would like to thank the members of the McNamara Lab for all of their feedback, encouragement and guidance. Specifically, thanks to Megan Mazzei for engaging in thoughtful discussions about the conflicting effects of G6PD-d on immune cells, and Tanmoy Mondal for his thoughts on the effect of G6PD-d on T cells. All figures in this manuscript were created with BioRender.com (Created in BioRender. Andrews, P. (2025) https://BioRender.com/gcvgl1y). P.H.A. and C.A.M. received funding from the LeDucq Foundation Transatlantic Network of Excellence: Checkpoint Athero and B cells and Cardiovascular Disease. C.A.M. is also funded by NIH R01AI172112, NIH P01 HL136275, and UVA SIF Immunology, Imaging and Informatics for Precision Immunomedicine in CVD. This review was written without direct funding.
Author information
Authors and Affiliations
Contributions
P.H.A. wrote the main manuscript text and prepared all figures with input and guidance from both C.A.M. and J.C.Z. All authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Andrews, P.H., Zimring, J.C. & McNamara, C.A. Clinical associations and potential cellular mechanisms linking G6PD deficiency and atherosclerotic cardiovascular disease. npj Metab Health Dis 3, 16 (2025). https://doi.org/10.1038/s44324-025-00061-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44324-025-00061-6