Abstract
This study aimed to determine whether daily low-dose aspirin reduces the risk of type 2 diabetes (T2D) associated with COVID-19. A longitudinal cohort of 200,000 adults followed from 2018 to 2022 was analyzed, comparing T2D incidence between aspirin users and non-users. Propensity score matching was used to balance the groups. The incidence of T2D was substantially lower in the aspirin group, with Cox regression showing a 52% risk reduction. Kaplan-Meier analysis confirmed a significant divergence in cumulative T2D risk after two years. This protective effect was observed both before and during the COVID-19 pandemic, with a stronger association during the pandemic period. These findings indicate that daily low-dose aspirin significantly reduces the risk of COVID-19-associated new-onset T2D, highlighting the role of inflammation in the pathogenesis of T2D triggered or unmasked by COVID-19.
Similar content being viewed by others
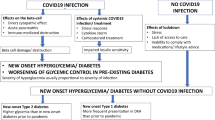
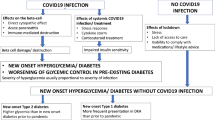
Substantial evidence has shown that the incidence of type 2 diabetes (T2D) increased during the acute phase of COVID-19 pandemic1,2,3,4,5,6,7,8,9,10,11,12,13. Initial reports compared groups of patients who certainly had a positive SARS-CoV-2 test vs individuals with similar demographic characteristics who did not have COVID-19, showing the effect of the long-term individual COVID-19 infection (post-acute sequelae) on the incidence of diabetes [reviewed in ref. 3].
Recently, we analyzed a large real-world dataset of adults and demonstrated that the incidence of newly diagnosed T2D in the general population was 4.85 per 1000 person-years before the COVID-19 pandemic, vs 12.21 per 1000 person-years during the pandemic14. However, these findings do not allow any conclusion on the pathophysiologic mechanisms underlying the increased incidence of T2D in the pandemic period, which could be ascribed to direct effects of SARS-CoV-2 infection, and indirect effects like stress, changes in diet/exercise and in cardiovascular prevention strategies, as well as reduced access to healthcare15,16,17,18.
The observation that fully vaccinated individuals might be protected from the risk of incident diabetes following SARS-CoV-2 infection19 seems to support a functional role of inflammation, autoimmune dysregulation, and endothelial dysfunction in the regulation of this process20,21,22,23,24,25,26,27,28. Indeed, experimental and epidemiological data have suggested that subclinical inflammation might contribute to metabolic diseases, insulin resistance, and T2D29,30. Specifically, analyzing the comprehensive data collected in the ASPREE trial31, Zoungas and coworkers32 have tested the hypothesis that treatment of healthy elder adults with 100 mg daily of enteric-coated oral aspirin would reduce incident diabetes or slow the increase in fasting glucose plasma concentration over time when compared with treatment with placebo. This post hoc analysis32 revealed that, during a median follow-up of 4,7 years, the aspirin group had a 15% reduction in risk of incident diabetes (hazard ratio 0·85 [95% CI: 0.75–0.97]; p = 0.013) compared to placebo, thus supporting the potential for anti-inflammatory agents such as aspirin to prevent T2D, at least in older adults, in which the mechanisms underlying the increased risk of new-onset T2D can differ from those observed at younger ages33,34,35,36.
Hence, in light of a possible role of inflammation in COVID-19-induced increased incidence of new diagnosed T2D14, in the present study we specifically analyzed the effect of daily low dose aspirin on incident T2D in a population closely monitored from 2018 to 2022. We juxtaposed these findings with data from a cohort not treated with aspirin. The main outcome of the study was the new diagnosis of T2D.
Results
Demographic and clinical characteristics of our population
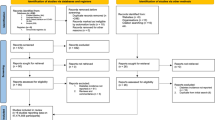
Anonymized data from electronic records of 247,975 patients in the 2018–2022 period were considered for the study. After excluding individuals not meeting our inclusion/exclusion criteria (please see methods), we obtained data on a cohort of 35,525 participants.
The demographic and clinical characteristics are presented in Supplementary Table 1. The aspirin treated population was older and with a higher value of BMI; in addition, it had a larger percentage of subjects with prediabetes or dyslipidemia or treated with statins or antihypertensive drugs.
Propensity score matching
Given the difference between individuals treated with aspirin and the control group in terms of baseline characteristics, propensity scores were calculated and used to match the two groups according to the baseline covariates [i.e., age, gender, and BMI]. The main characteristics of these groups after weighting are shown in Table 1. Evaluation of standardized mean differences of these characteristics after weighting revealed no significant difference, suggesting good balance. At the final follow-up visit, concomitant medication use, including anti-hypertensive drug classes, statins, and other lipid-lowering medications, was comparable between the aspirin and the non-aspirin groups.
During the follow-up, 999 (12% of the study population) incident T2D events were reported, including 330 in the aspirin group (15.9 cases per 1000 person-years) and 664 in the non-aspirin group (32 cases per 1000 person-years).
As shown in Table 2, the incidence of new-onset T2D was significantly lower in subjects treated with aspirin. However, considering that the incidence of comorbidity was different between the two groups, we performed a Cox model on the total follow-up period demonstrating a significant protective effect of aspirin, since assignment to aspirin resulted in a 52% reduction in risk of incident T2D (HR = 0.48 95% CI: 0,42–0.45, p < 0.001). Strikingly, Kaplan–Meier curves confirmed a significantly different cumulative risk of T2D for the treated and not treated groups, with a divergence in case numbers of T2D beginning after the second year of aspirin treatment (Fig. 1), with an evident progressive increase in the difference between the curves that plotted the cumulative incidence of T2D (log-rank test p < 0.001).
Daily low-dose aspirin reduces the risk of COVID-19-associated new-onset T2D
In order to assess whether there was any difference in the protective effect of aspirin on the incidence of new T2D before and during the pandemic, we divided the study period into two parts and performed two separate Cox and Kaplan–Meier analyses. The Cox model of the pre-pandemic period demonstrated a HR of new-onset T2D in the aspirin treated group of 0.71 (95% CI: 0.56–0.89, p < 0.003) and a difference between the Kaplan–Meier curves of the two study groups, which achieved statistical significance (p < 0.025; Fig. 2A). During the COVID-19 pandemic, the treatment with aspirin reduced the HR of new-onset T2D to 0.38 (95% CI 0.32.0.35, p < 001). Even in this study period, Kaplan–Meier curves plotting the cumulative incidence of new T2D in the aspirin treated and control groups were statistically different (p < 0.0001), starting to diverge very early displaying a difference that progressively increased, so that at the end of the observation period the cumulative risk of new-onset TD2 in the aspirin treated patients was less than half of that of the control group (Fig. 2B).
Safety analysis
The safety analysis set included 8278 participants, with 4139 in the aspirin group and 4139 in the non-aspirin group. Major bleeding occurred in 9 (0.3%) participants in the aspirin group and 3 (0.1%) participants in the non-aspirin group. Moderate bleeding had an incidence rate of 8.3 events per 1000 person-years in the aspirin group and 4.2 events per 1000 person-years in the non-aspirin group. Finally, minor bleeding events, represented by hematuria, were 6.7 per 1000 person-years in the aspirin group and 4.9 per 1000 person-years in the non-aspirin group. Compared with non-aspirin, aspirin treatment increased the risk of bleeding, including intracranial, gastrointestinal, and other clinically significant bleeding.
Discussion
To the best of our knowledge, our study is the first to be performed on a large sample of the general population and not on post hoc analyses of trials designed to address different primary and secondary outcomes, or databases of hospitals or outpatient clinics. Indeed, in Italy, all citizens have a primary care physician (family doctor) who follows them even if they do not have specific pathologies. Therefore, the database that we used37 includes not only patients with known illnesses but also individuals who do not have any disease (or at least are not aware of it).
Thus, the finding that a daily low dose of aspirin reduces the risk of developing T2D seems noteworthy for the management of the progressive increase of this disease.
Our finding of a 15.9/1000 person-years incidence of new T2D in the control population may appear in contrast with the 14.8 reported by Zoungas32, but we should consider: (a) the high percentage of subjects with prediabetes for which has been reported an incidence of new T2D of 21.7% in three years of follow-up14; (b) the increase of about twice and half of the rate of new T2D incidence during the pandemic14,38. Interestingly, the cumulative reduction of ~50% of the risk of new-onset T2D observed in our study derives from a 29% reduction in the pre-pandemic period and a 62% reduction during COVID-19.
A cytokine storm in people infected with SARS-CoV-2 is a prothrombotic, highly inflammatory pathological state that may have direct and indirect effects on pancreatic beta-cells39,40,41,42,43,44,45,46,47. In fact, the current available evidence on the actual expression of ACE2 in human islets is controversial: some authors have reported the expression of ACE2 in islets39,48,49, whereas these findings have not been confirmed by others40,41,42,50,51,52,53,54. Yet, evidence from COVID-19 patients indicates a significant increase in pro-inflammatory cytokines, suggesting that inflammation may play a crucial role in the impaired glucose homeostasis observed in COVID-19 patients55. The elevated levels of pro-inflammatory cytokines and acute-phase reactants triggered by COVID-19 may contribute to direct inflammation and injury of pancreatic β-cells. Individuals with acute SARS-CoV-2 infection can develop a cytokine storm, a severe inflammatory response that impacts multiple organs, including the pancreas; this heightened inflammatory state may lead to acute pancreatitis and deterioration of pancreatic islet cells25,56,57. Taken together, these observations corroborate the hypothesis that inflammation may play a pivotal role in the development of T2D in COVID-19-infected patients.
The anti-inflammatory effects of aspirin have been proposed as a possible mechanism underlying its ability to reduce new-onset T2D58,59. In this regard, Zoungas et al32. considered that preclinical and clinical studies have provided a good rationale for targeting inflammation to improve the action of insulin and improve blood glucose levels60. In particular, studies of salicylate treatment have reported anti-inflammatory effects at both low and high doses, albeit through different mechanisms of action. In an experimental model of cantharidin-induced acute inflammation, low-dose aspirin (75 mg) administered for 10 days to healthy men inhibited innate immune-mediated responses by reducing total leukocyte as well as neutrophil and macrophage accumulation in skin blisters. These effects were dependent on 15-epi-lipoxin A4 synthesis and signaling, which triggered anti-adhesive nitric oxide release, a crucial determinant of extravascular leukocyte accumulation and inhibition of prostacyclin production through the cyclooxygenase pathway61. In contrast, high-dose salicylates, such as salsalate (the non-acetylated dimer of salicylic acid), have been shown to be potent inhibitors of IκB kinase and the NF-κB cascade62. In studies of high-risk populations with prediabetes or populations with diabetes, high-dose salsalate improved insulin sensitivity, as well as increased insulin release and peripheral glucose disposal63,64,65,66.
In light of these considerations, we were able to mirror in a real-life registry the beneficial effect of aspirin on T2D development demonstrated in experimental models; the observation that during COVID-19 subjects treated with low daily dose of aspirin led to a reduction of the risk to develop new-onset T2D almost double as compared to the pre-pandemic period corroborates the responsibility of inflammation in the genesis of the marked increase in the rate of new T2D during COVID-19 pandemic. This finding is also substantiated by the results of the 10-year randomized double-blind placebo-controlled Women’s Health Study, which suggests that long-term low-dose aspirin does not prevent the development of T2D67.
We also report an increased risk of major bleeding, which is an important consideration for subjects in primary prevention. The magnitude of this increased risk was commensurate with that reported by Zoungas32 and other low-dose aspirin trials68,69. However, we included bleeding episodes of lesser severity which may have significant impact for patients, but are difficult to be captured and were not considered in previous studies. As a consequence, the component of severe and moderate bleedings was lower than those recorded in studies which included patients in secondary prevention.
Our study is not exempt from limitations. For instance, we do not have data on inflammatory markers or modifications of lifestyle behaviors including physical activity, dietary habits, and sleeping patterns, which could have played a role in increasing the risk of diabetes after COVID-1913,70; we also do not have full data on the severity of COVID-19 or on vaccination, which has been shown to reduce the severity and long-term effects of COVID-19, including the increase in the onset of new diabetes71,72. Nonetheless, the large sample size of participants, the long follow-up with detailed data capture, alongside the large number of incident diabetes cases, gives us confidence that the effects were robust.
Taken together, our data indicate that a daily low dose of aspirin significantly reduces the risk of new-onset T2D associated with COVID-19. The observation that aspirin reduces the incidence of new T2D but increase bleeding risk does not endorse to use such a therapy for diabetes prevention; however, the particularly marked effect on the risk to develop diabetes during the COVID-19 pandemic may warrant further studies.
Methods
Study design
We conducted a longitudinal cohort study utilizing data from COMEGEN, a cooperative of general practitioners operating within the Naples Local Health Authority of the Italian Ministry of Health (ASL Napoli 1 Centro). Established in 1997, COMEGEN now comprises 140 physicians who are interconnected through a shared digital medical record system, generating a comprehensive repository of health data for over 200,000 adults in Italy37. Each physician updates medical records daily, documenting all aspects of outpatient care. The demographic distribution of patients aligns with the city’s population as recorded by the National Institute of Statistics (ISTAT), with no significant variations in geographic representation or age stratification. Diagnoses are classified according to the International Classification of Diseases 10 (ICD-X) and standardized coding is applied to all prescribed diagnostic tests. Pharmaceutical prescriptions are systematically recorded, including details on the date, brand name, active ingredients, dosage, and administration guidelines. Additionally, the database encompasses information on vital parameters, anthropometric measures, chronic conditions, medical consultations, hospital admissions, emergency visits, prescription drug dispensations, laboratory tests, and mortality data. This extensive dataset facilitates real-time monitoring of patient management, encompassing treatment processes, clinical outcomes, medication use, diagnostic procedures, and the overall complexity and comorbidity burden of the patient population37. Precise person-time assessment is fundamental for calculating incidence rates, and COMEGEN enables accurate tracking by documenting the enrollment date of each patient, the start of data contribution, and the dates marking death, follow-up completion, or study termination.
We collected data from January 1st, 2018, to December 31st, 2022. The COMEGEN database provided demographic and clinical data. Laboratory measurements and medication data were available, as well. Information on COVID-19 was obtained from the Campania Region COVID-19 shared data resource. The diagnosis of COVID-19 was confirmed by the documentation of a positive polymerase chain reaction (PCR) test, as we previously described73,74,75.
The Ethics Board at the “ASL Napoli 1 Centro” reviewed and approved this study and granted a waiver of informed consent (Protocol #257/22–23). The investigation conforms to the principles outlined in the Declaration of Helsinki, including its later amendments.
The exposure was the beginning of daily low-dose aspirin (100 mg) treatment, and the primary outcome was newly diagnosed T2D. At the beginning of the observation period, we removed from the study cohort all individuals with a record of HbA1c > 6.5% (48 mmol/mol), or a previous diagnosis of diabetes as defined by the ICD-10 codes (E08.X to E13.X), or prescription record of antidiabetic medications for more than 30 days. Only individuals whose information about T2D history was entirely available for the 5 years and for whom selected demographic and clinical data were available for the entire observation period were included in the study. For this reason, although individuals were able to join the cohort over time as they became eligible, they did so in only limited numbers.
Inclusion criteria
Age >18 years old; availability of information on diabetes history; availability of clinical and demographic data at least for the 2 years before or the 3 years after the COVID-19 outbreak.
Exclusion criteria
History of diabetes (both T2D or T1D) or chronic kidney disease before 2018, cardiac ischemia, myocardial infarction, stroke, any site hemorrhage, heart failure, other prescriptions of antiplatelet therapy, missing information on history, clinical, or demographic data; people who were already on aspirin prior to the study period were excluded as well.
Variables
In order to adjust for the difference in baseline characteristics between groups, we used clinically available predefined variables, which were selected based on a previous report76 where they were used to define a diabetic risk score. Predefined baseline variables included age, sex, body mass index (BMI), and blood glucose measurements. Comorbidities such as prediabetes, cardiovascular disease, chronic kidney disease, hypertension, and dyslipidemia were also included as predefined covariates since it has been reported that patients with cardiovascular disease, arterial hypertension, hyperlipidemia, or prediabetes exhibit higher risks and burdens than people without these conditions76,77.
Outcomes
The main outcome of the study was the new diagnosis of T2D, assessed by the ICD-X codes E11 (“T2D”) and E13X (“Other specified diabetes mellitus”) with prescription of antidiabetic therapies for more than 30 days. Diagnoses of T1D, including the specific code E08, were excluded.
Bleeding represented the safety outcome. We created a binary outcome based on an observation of at least one bleeding event or no recorded bleeding event as of the last available assessment. This outcome was designed to provide a conservative estimate of bleeding events during the follow-up. The involvement of the primary physicians also allows a reliable estimate of bleeding episodes of lesser severity, which may have a significant impact on patients, but are difficult to capture. Bleeding complications were classified as severe or life-threatening if they were intracerebral or if they resulted in substantial hemodynamic compromise requiring treatment. Moderate bleeding was defined by the need for transfusion. Minor bleeding referred to other bleedings, not requiring transfusion or causing hemodynamic compromise, according to a previously reported bleeding classification78.
Statistical analysis
Data were summarized using mean and standard deviation for continuous variables and absolute frequency with percentage for categorical variables. A propensity score model was implemented to achieve matching of the 35,525 individuals, of which 5066 were under oral aspirin therapy and 30459 without this therapy, considering the latter as the control group. The matching was conducted as we previously described37,79,80, with the following covariates: age, sex, BMI, statin therapy, antihypertensive therapy and prediabetes. The optimal matching was achieved using nearest neighbor matching with logit link and a caliper = 0.1, with a 1:1 ratio without replacement79. Mean differences were reported as Cohen’s D. Excess risk is computed as the difference between proportions for two independent populations. All differences are reported with 95% confidence intervals. Cox regression was used to identify the association of aspirin with diabetes, considered as a time-to-event variable. The proportional hazard assumption was tested using the Schoenfeld residuals. The cumulative risk curves were constructed using the Kaplan–Meier method, and a comparison between curves is computed with the log-rank test. Sensitivity analyses included the removal of all outliers and the exclusion of covariates with more than 30% missing values. Analyses were performed with R statistical software version 4.4.0. A p-value < 0.05 was considered significant for all analyses.
Data availability
The data underlying this article will be shared on reasonable request to the first authors.
References
Stiegmann, R. A., Payne, C. B., Kiel, M. A. & Stahlman, S. L. Increased prevalence of overweight and obesity and incidence of prediabetes and type 2 diabetes during the COVID-19 Pandemic, Active Component Service Members, U.S. Armed Forces, 2018 to 2021. MSMR 30, 11–18 (2023).
Li, J. et al. Increased risk of new-onset diabetes in patients with COVID-19: a systematic review and meta-analysis. Front. Public Health 11, 1170156 (2023).
Cefalu, W. T. COVID-19 and rising incidence of diabetes: despite evolving data, an enigma still to be solved. Diab. Care 46, 913–915 (2023).
Bellia, C. et al. Prevalence and risk of new-onset diabetes mellitus after COVID-19: a systematic review and meta-analysis. Front. Endocrinol. ((Lausanne)) 14, 1215879 (2023).
Miller, M. G., Terebuh, P., Kaelber, D. C., Xu, R. & Davis, P. B. SARS-CoV-2 Infection and New-Onset Type 2 Diabetes Among Pediatric Patients, 2020 to 2022. JAMA Netw Open. 7, e2439444 (2024).
Accili, D. Can COVID-19 cause diabetes?. Nat. Metab. 3, 123–125 (2021).
Rubino, F. et al. New-onset diabetes in Covid-19. N. Engl. J. Med. 383, 789–790 (2020).
Choi, J. H., Kim, K. M., Song, K. & Seo, G. H. Risk for newly diagnosed type 2 diabetes mellitus after COVID-19 among Korean adults: a Nationwide Matched Cohort Study. Endocrinol. Metab. ((Seoul.)) 38, 245–252 (2023).
Lai, H. et al. Risk of incident diabetes after COVID-19 infection: a systematic review and meta-analysis. Metabolism 137, 155330 (2022).
Birabaharan, M., Kaelber, D. C., Pettus, J. H. & Smith, D. M. Risk of new-onset type 2 diabetes in 600 055 people after COVID-19: a cohort study. Diab. Obes. Metab. 24, 1176–1179 (2022).
Vas, P., Hopkins, D., Feher, M., Rubino, F. & B Whyte, M. Diabetes, obesity and COVID-19: a complex interplay. Diab. Obes. Metab. 22, 1892–1896 (2020).
O’Mahoney, L. L. et al. The prevalence and long-term health effects of Long Covid among hospitalised and non-hospitalised populations: a systematic review and meta-analysis. EClinicalMedicine 55, 101762 (2023).
Kim, S. H. et al. New-Onset Diabetes After COVID-19. J. Clin. Endocrinol. Metab. 108, e1164–e1174 (2023).
Izzo, R. et al. Incidence of type 2 diabetes before and during the COVID-19 pandemic in Naples, Italy: a longitudinal cohort study. EClinicalMedicine 66, 102345 (2023).
Pantea Stoian, A. et al. Cardiometabolic panel of international experts on syndemic C: new-onset diabetes mellitus in COVID-19: a scoping review. Diab. Ther. 15, 33–60 (2024).
Drucker, D. J. Diabetes, obesity, metabolism, and SARS-CoV-2 infection: the end of the beginning. Cell Metab. 33, 479–498 (2021).
Khunti, K. et al. COVID-19, hyperglycemia, and new-onset diabetes. Diab. Care 44, 2645–2655 (2021).
Landstra, C. P. & de Koning, E. J. P. COVID-19 and diabetes: understanding the interrelationship and risks for a severe course. Front. Endocrinol. ((Lausanne)) 12, 649525 (2021).
Xiong, X. et al. Incidence of diabetes following COVID-19 vaccination and SARS-CoV-2 infection in Hong Kong: a population-based cohort study. PLoS Med. 20, e1004274 (2023).
Kazakou, P. et al. Diabetes and COVID-19: a bidirectional interplay. Front. Endocrinol. (Lausanne) 13, 780663 (2022).
Bansal, R., Gubbi, S. & Muniyappa, R. Metabolic syndrome and COVID 19: endocrine-immune-vascular interactions shapes clinical course. Endocrinology 161, bqaa112 (2020).
Johnson, J. E. et al. Coronavirus disease 2019 (COVID-19) coronary vascular thrombosis: correlation with neutrophil but not endothelial activation. Am. J. Pathol. 192, 112–120 (2022).
Sardu, C. et al. Hypertension, Thrombosis, Kidney Failure, and Diabetes: Is COVID-19 an Endothelial Disease? A Comprehensive Evaluation of Clinical and Basic Evidence. J Clin Med 9, 1417 (2020).
Jankauskas, S. S. et al. COVID-19 causes ferroptosis and oxidative stress in human endothelial cells. Antioxidants (Basel) 12, 326 (2023).
Grubisic, B. et al. Molecular mechanisms responsible for diabetogenic effects of COVID-19 infection-induction of autoimmune dysregulation and metabolic disturbances. Int. J. Mol. Sci. 24, 11576 (2023).
Di Pietro, P. et al. Plasma miR-1-3p levels predict severity in hospitalized COVID-19 patients. Br. J. Pharm. 182, 451–467 (2025).
El-Naas, A. et al. New onset of type 1 and type 2 diabetes post-COVID-19 infection: a systematic review. Emerg. Microbes Infect. https://doi.org/10.1080/22221751.2025.2492211 (2025).
O’Mahoney, L. L. et al. The risk of Long Covid symptoms: a systematic review and meta-analysis of controlled studies. Nat. Commun. 16, 4249 (2025).
Oguntibeju, O. O. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int. J. Physiol. Pathophysiol. Pharm. 11, 45–63 (2019).
Weinberg Sibony, R., Segev, O., Dor, S. & Raz, I. Overview of oxidative stress and inflammation in diabetes. J. Diab. 16, e70014 (2024).
McNeil, J. J. et al. Effect of aspirin on all-cause mortality in the healthy elderly. N. Engl. J. Med. 379, 1519–1528 (2018).
Zoungas, S. et al. Daily low-dose aspirin and incident type 2 diabetes in community-dwelling healthy older adults: a post-hoc analysis of efficacy and safety in the ASPREE randomised placebo-controlled trial. Lancet Diab. Endocrinol. 12, 98–106 (2024).
Greenfield, J. R. & Campbell, L. V. Relationship between inflammation, insulin resistance and type 2 diabetes: ‘cause or effect’?. Curr. Diab. Rev. 2, 195–211 (2006).
Zeyfang, A., Wernecke, J. & Bahrmann, A. Diabetes mellitus at an elderly age. Exp. Clin. Endocrinol. Diabetes 133, 168–176 (2025).
Lee, P. G. & Halter, J. B. The pathophysiology of hyperglycemia in older adults: clinical considerations. Diab. Care 40, 444–452 (2017).
Mone, P. et al. Cognitive impairment in frail hypertensive elderly patients: role of hyperglycemia. Cells 10, 2115 (2021).
Trimarco, V. et al. A six-year study in a real-world population reveals an increased incidence of dyslipidemia during COVID-19. J. Clin. Invest. 134, e183777 (2024).
Trimarco, V. et al. Increased prevalence of cardiovascular-kidney-metabolic syndrome during COVID-19: a propensity score-matched study. Diab. Res. Clin. Pract. 218, 111926 (2024).
Fignani, D. et al. SARS-CoV-2 receptor angiotensin I-converting enzyme type 2 (ACE2) is expressed in human pancreatic beta-cells and in the human pancreas microvasculature. Front. Endocrinol. (Lausanne) 11, 596898 (2020).
Kusmartseva, I. et al. Expression of SARS-CoV-2 entry factors in the pancreas of normal organ donors and individuals with COVID-19. Cell Metab. 32, 1041–1051.e1046 (2020).
Qadir, M. M. F. et al. SARS-CoV-2 infection of the pancreas promotes thrombofibrosis and is associated with new-onset diabetes. JCI Insight 6, e151551 (2021).
Clark, A. L. & Mirmira, R. G. SARS-CoV-2 infection of islet beta cells: evidence and implications. Cell Rep. Med. 2, 100380 (2021).
Atkinson, M. A. & Powers, A. C. Distinguishing the real from the hyperglycaemia: does COVID-19 induce diabetes?. Lancet Diab. Endocrinol. 9, 328–329 (2021).
Steenblock, C. et al. Viral infiltration of pancreatic islets in patients with COVID-19. Nat. Commun. 12, 3534 (2021).
Shirakawa, J. Pancreatic beta-cell fate in subjects with COVID-19. J. Diab. Investig. 12, 2126–2128 (2021).
El-Huneidi, W., Hamad, M. & Taneera, J. Expression of SARS-CoV-2 receptor “ACE2” in human pancreatic beta cells: to be or not to be!. Islets 13, 106–114 (2021).
Morris, A. Effects of pancreatic SARS-CoV-2 infection identified. Nat. Rev. Endocrinol. 17, 192 (2021).
Muller, J. A. et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat. Metab. 3, 149–165 (2021).
Wu, C. T. et al. SARS-CoV-2 infects human pancreatic beta cells and elicits beta cell impairment. Cell Metab. 33, 1565–1576.e1565 (2021).
Coate, K. C. et al. SARS-CoV-2 cell entry factors ACE2 and TMPRSS2 are expressed in the microvasculature and ducts of human pancreas but are not enriched in beta cells. Cell Metab. 32, 1028–1040.e1024 (2020).
Segerstolpe, A. et al. Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metab. 24, 593–607 (2016).
Blodgett, D. M. et al. Novel observations from next-generation RNA sequencing of highly purified human adult and fetal islet cell subsets. Diabetes 64, 3172–3181 (2015).
Baron, M. et al. A single-cell transcriptomic map of the human and mouse pancreas reveals inter- and intra-cell population structure. Cell Syst. 3, 346–360.e344 (2016).
Arda, H. E. et al. Age-dependent pancreatic gene regulation reveals mechanisms governing human beta cell function. Cell Metab. 23, 909–920 (2016).
D’Ardes, D. et al. Metabolic changes in SARS-CoV-2 infection: clinical data and molecular hypothesis to explain alterations of lipid profile and thyroid function observed in COVID-19 patients. Life (Basel) 11, 860 (2021).
Ahlqvist, E. et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diab. Endocrinol. 6, 361–369 (2018).
Holshue, M. L. et al. Washington state -nCo VCIT: first case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 382, 929–936 (2020).
Lembo, M. et al. Daily low dose aspirin halves incident type 2 diabetes in elderly subjects with prediabetes. A five-year longitudinal cohort study in a real-word population. Cardiovasc Diabetol. 2025; in press.
Pellegrini, V. et al. Inflammatory trajectory of type 2 diabetes: novel opportunities for early and late treatment. Cells 13, 1662 (2024).
Rumore, M. M. & Kim, K. S. Potential role of salicylates in type 2 diabetes. Ann. Pharmacother. 44, 1207–1221 (2010).
Morris, T. et al. Effects of low-dose aspirin on acute inflammatory responses in humans. J. Immunol. 183, 2089–2096 (2009).
Shoelson, S. E., Lee, J. & Yuan, M. Inflammation and the IKK beta/I kappa B/NF-kappa B axis in obesity- and diet-induced insulin resistance. Int J. Obes. Relat. Metab. Disord. 27, S49–S52 (2003).
Manrique, C., Lastra, G., Palmer, J., Gardner, M. & Sowers, J. R. Aspirin and diabetes mellitus: revisiting an old player. Ther. Adv. Cardiovasc Dis. 2, 37–42 (2008).
Lastra, G. & Whaley-Connell, A. Diabetes: aspirin and prevention of diabetes still a topic of debate. Nat. Rev. Endocrinol. 5, 365–366 (2009).
Faghihimani, E. et al. Reduction of insulin resistance and plasma glucose level by salsalate treatment in persons with prediabetes. Endocr. Pract. 18, 826–833 (2012).
Fleischman, A., Shoelson, S. E., Bernier, R. & Goldfine, A. B. Salsalate improves glycemia and inflammatory parameters in obese young adults. Diab. Care 31, 289–294 (2008).
Pradhan, A. D., Cook, N. R., Manson, J. E., Ridker, P. M. & Buring, J. E. A randomized trial of low-dose aspirin in the prevention of clinical type 2 diabetes in women. Diab. Care 32, 3–8 (2009).
Group, A. S. C. et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N. Engl. J. Med. 379, 1529–1539 (2018).
Gaziano, J. M. et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet 392, 1036–1046 (2018).
Erthal, I. N. et al. Lifestyle pattern changes, eating disorders, and sleep quality in diabetes: how are the effects of 18 months of COVID-19 pandemic being felt?. Acta Diabetol. 59, 1265–1274 (2022).
Tran, V. T., Perrodeau, E., Saldanha, J., Pane, I. & Ravaud, P. Efficacy of first dose of COVID-19 vaccine versus no vaccination on symptoms of patients with long covid: target trial emulation based on ComPaRe e-cohort. BMJ Med. 2, e000229 (2023).
Notarte, K. I. et al. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: a systematic review. EClinicalMedicine 53, 101624 (2022).
Mone, P. et al. Endothelial extracellular vesicles enriched in microRNA-34a predict new-onset diabetes in coronavirus disease 2019 (COVID-19) patients: novel insights for long COVID metabolic sequelae. J. Pharm. Exp. Ther. 389, 34–39 (2024).
Fiorentino, G. et al. Effects of adding L-arginine orally to standard therapy in patients with COVID-19: a randomized, double-blind, placebo-controlled, parallel-group trial. Results of the first interim analysis. EClinicalMedicine 40, 101125 (2021).
Gambardella, J. et al. Role of endothelial miR-24 in COVID-19 cerebrovascular events. Crit. Care 25, 306 (2021).
Xie, Y. & Al-Aly, Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diab. Endocrinol. 10, 311–321 (2022).
Joseph, J. J. et al. Comprehensive management of cardiovascular risk factors for adults with type 2 diabetes: a scientific statement from the American Heart Association. Circulation 145, e722–e759 (2022).
Mehran, R. et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 123, 2736–2747 (2011).
Mone, P. et al. Stress hyperglycemia drives the risk of hospitalization for chest pain in patients with ischemia and nonobstructive coronary arteries (INOCA). Diab. Care 46, 450–454 (2023).
Lembo, M. et al. Statin-induced risk of diabetes does not reduce cardiovascular benefits in primary prevention: a 6-year propensity-score matched study in a large population. Cardiovasc Diabetol 24, 233 (2025).
Acknowledgements
The Santulli’s Lab is currently supported in part by the National Institutes of Health (NIH): National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK: R01-DK123259, R01-DK033823), National Heart, Lung, and Blood Institute (NHLBI: R01-HL164772, R01-HL146691, R01-HL159062, T32-HL144456, T32-HL172255), National Center for Advancing Translational Sciences (NCATS: UM1-TR004400, UL1-TR002556-06) to G.S., by the American Heart Association (AHA, 24IPA1268813), and by the Monique Weill-Caulier and Irma T. Hirschl Trusts (to G.S.). The work was also supported by PNRR-POC-2022-12376833 (CAREMODE Project: New multimodal CArdioREspiratory MOnitoring DEvice to improve chronic patient management), financed by the European Union (EU), NextGenerationEU—CUP: C63C22001310007 to R.I.
Author information
Authors and Affiliations
Contributions
G.S. and B.T. conceived the idea; V.T., R.I., M.V.M., and B.T. wrote the first draft of the paper; D.P. performed statistical analysis; S.S.J., P.G., F.R., G.G., A.S., G.E., R.P., G.P., C.M., and M.L. helped in collecting and analyzing data; G.S. revised critically the paper. V.T., R.I., D.P., and M.V.M. are co-first authors; G.S. and B.T. are co-last authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Trimarco, V., Izzo, R., Pacella, D. et al. Aspirin reduces the risk of type 2 diabetes associated with COVID-19. npj Metab Health Dis 3, 27 (2025). https://doi.org/10.1038/s44324-025-00072-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s44324-025-00072-3
This article is cited by
-
A six-year longitudinal study identifies a statin-independent association between low LDL-cholesterol and risk of type 2 diabetes
Cardiovascular Diabetology (2025)