Abstract
Hepatocellular carcinoma (HCC), the third cause of cancer-related deaths worldwide, continues to present significant therapeutic challenges. Despite significant therapeutic advancement in the last decade, the efficacy of systemic treatments for patients with advanced HCC remains unsatisfactory. While the clinical introduction of immune checkpoint inhibitors has improved response rates and overall survival, their clinical success remains still limited. Here we provide a comprehensive review of current and emerging strategies aimed at enhancing the efficacy of immunotherapy for the treatment of HCC. Both clinical studies conducted in patients as well as studies in preclinical models have markedly advanced our understanding of resistance as well as uncovered novel approaches to overcome resistance. Recent progress is paving the way for improved efficacy and safety of novel approaches that will improve the dismal prognosis of patients with advanced HCC.
Similar content being viewed by others
Unmet medical need to treat advanced liver cancer
Liver cancer is one of the most commonly diagnosed cancers and the third main cause of cancer-related deaths worldwide, with >900,000 new cases and >800,000 deaths in 20201. Globally, the annual number of new cases of liver cancer is predicted to increase, with 1.4 million people projected to be diagnosed in 20402. Progress in antiviral therapy combined with increased incidence in metabolic diseases leads to an etiological shift from virus-induced to metabolic-associated liver cancer within the next decades3,4. Hepatocellular carcinoma (HCC), accounting for 90% of liver cancers, is still difficult to treat. Key reasons include: (1) its development on a background of advanced chronic liver diseases in most cases, (2) its phenotypic, genomic, and molecular heterogeneity, and (3) late-stage detection due to absent or limited clinical symptoms at the early stages5,6. Early-stage (Barcelona Clinic Liver Cancer (BCLC) class A) patients can undergo surgical resection, percutaneous ablation, or liver transplantation, which can be considered curative, in well-selected patients, and yield median overall survival (mOS) of >5 and >10 years, respectively7,8.
Following the approval of sorafenib around 15 years ago9, marked progress has been made in the treatment of advanced HCC. Several novel treatment regimens have been approved4. The latest AASLD guidelines10 recommend the use of the atezolizumab plus bevacizumab combination; or tremelimumab plus durvalumab, as first line. Sorafenib, Lenvatinib or Durvalumab are alternatives in case of contraindications. Atezolizumab, durvalumab, and tremelimumab are immune checkpoints inhibitors (ICIs), the first two targeting programmed cell death ligand 1 (PD-L1) and the latest cytotoxic T-lymphocyte-associated protein 4 (CTLA-4). The two above mentioned combinations showed improved survival over sorafenib, with a mOS of 19.2 and 16.43 months, respectively11,12,13. However, regardless of the poor survival rate of these patients under treatment, the response rate is limited, with an objective response rate (ORR) of only 25% in both studies and a complete response in only 5% and 2% of patients respectively. Despite a significant improvement in patient survival, the response rate of ICIs remains unsatisfactory. To identify new therapeutic targets, it is critical to unveil the mechanisms of resistance to immunotherapy. Herein, we explore the known mechanism of resistance to ICIs and present the results of preclinical models using combination therapy to thwart ICI resistance.
Immune checkpoint inhibitors
The PD-1/PD-L1 pathway is a crucial regulator of immune tolerance within the tumor microenvironment. PD-1 is a type 1 transmembrane protein belonging to the CD28 immunoglobulin family. Structurally, it comprises a 288-amino-acid-long protein with an extracellular Ig-V-like N-terminal domain, a hydrophobic transmembrane region, and a cytoplasmic tail characterized by two tyrosine residues14. These residues form an immune receptor tyrosine-based inhibitory motif (ITIM) and an immune receptor tyrosine-based switch motif (ITSM). It has been elucidated through mutagenetic studies that PD-1’s inhibitory effect on T cells is predominantly mediated via the activated ITSM15. PD-1 expression is not limited to T cells but extends to B cells, NK cells, monocytes, dendritic cells (DCs), and various tumor-infiltrating lymphocytes. Importantly, PD-1 is also expressed on regulatory T cells (Tregs), where it inhibits their proliferation and contributes to the immune response’s suppression16,17. The PD-1 pathway includes two ligands, PD-L1 and PD-L2, expressed on a range of cells, including pancreatic islet cells, vascular endothelium, and various antigen-presenting cells (APCs). PD-L1’s expression is particularly notable in multiple tumor types, including gastric cancers, melanomas, and HCC, making it a critical target in immunotherapy. PD-L1 binds to PD-1 with a lower affinity compared to PD-L218, yet its widespread expression on tumor cells and significant association with clinical outcomes in different cancers, including HCC19,20, made it a better therapeutic target for immunotherapy. Recently, studies discovered the capacity of the co-activator CD80 to form a heterodimer with PD-1 on the surface of APCs, which inhibits its capacity to bind PD-L1 and could explain part of the resistance to therapies targeting immune checkpoints21. The engagement of PD-1 with its ligands leads to the inhibition of T-cell activation, which ultimately leads to T-cell exhaustion and tumor progression22,23. This process is facilitated by several pro-inflammatory molecules, including interferon-gamma, which play a significant role in upregulating PD-L1 expression24. Monoclonal antibodies targeting PD-1 or PD-L1, such as nivolumab, atezolizumab, or durvalumab, effectively prevent the inhibitory signaling and reactivate T-cell-mediated cytotoxicity against tumor cells. They were approved as single agents or as part of combination therapy for the treatment of advanced HCC, either as first-line or second line, and numerous alternative antibodies are currently under clinical trial (see Table 1).
CTLA-4 is a transmembrane protein with a crucial role in regulating the amplitude of T-cell responses during the early priming phase in lymphoid organs. It is actually the first immune checkpoint protein to be discovered25, in 1987, and the first one to be successfully targeted with monoclonal antibody in a preclinical model of cancer26. Similar to PD-1, CTLA-4 is part of the CD28 immunoglobulin family and binds to B7 ligands (CD80 and CD86) on APCs. The expression of CTLA-4 is not detectable in naive T cells but is upregulated upon T-cell activation and is also expressed in Tregs. CTLA-4 outcompetes CD28 for B7 ligands, resulting in T-cell anergy27,28. In APCs, CTLA-4 binding results in reduced CD28 binding and impeded T-cell activation29. Moreover, CTLA-4 engagement has been shown to inhibit IL-2 production and T-cell proliferation and to induce cell cycle arrest30. This occurs through interaction with PP2A, SHP-2, and PI3K, which transduce downstream signaling that inhibits T-cell receptor signaling, together with the inhibition of other pathways linked to cell proliferation and survival, such as the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), PI3K, and MAP kinase pathways31,32,33. Furthermore, accumulating evidence points to an important role of CTLA-4 signaling in mediating the suppressive functions of Tregs. Anti-CTLA-4 antibodies bind to CTLA-4 molecules with high affinity, leading to Tregs functional blockade, resulting in enhanced T-cell activation and immunological responses to cancer34. CTLA-4 inhibitors have shown success in treating metastatic melanomas and are being explored for HCC treatment. CTLA-4 positive T cells and DCs are associated with impaired T-cell functionality35,36, and CTLA-4 inhibitors showed some potential in early clinical trials and ex-vivo models37,38. Importantly, CTLA-4 inhibition could have some synergetic effects with anti-PD-117. CTLA-4 inhibition by tremelimumab or ipilimumab showed some potential in phase II and phase III clinical trials, either as first or second-line therapy11,39. More, a virtual clinical trial of the nivolumab and ipilimumab combinations in >5000 virtual patients confirmed the therapeutic potential of CLTA-4 inhibition in HCC40. Numerous clinical trials are ongoing to test CLTA-4 inhibition in combination with other targets for the treatment of HCC (Table 1).
Approaches to improve the efficacy of ICIs
Among the different strategies to improve the efficacy of ICIs, one of the most promising is neoantigen vaccination. Neoantigens derive from proteins coding gene mutations or non-mutational sources such as RNA alternative splicing or epigenetic remodeling41. Tumor-associated antigens (TAA) are exclusive to tumors, they can bind to major histocompatibility complex (MHC) molecules and are recognized by T cells, triggering robust anti-tumor responses. Hence, TAA-based vaccines have the potential to be used alone or in combination with ICIs to trigger a potent antitumour immune response and potentially display some curative capacity. Several clinical trials are ongoing in different cancer types using this technology and some of them showed promising results42. Importantly, a recent study from Yarchoan and colleagues used a plasmid encoding a combination of 40 neoantigens in combination with pembrolizumab in 36 patients with advanced HCC, previously treated with multikinase inhibitor. The treatment showed no severe adverse effect, an ORR of ~30%, and ~8% of complete response43. These results, especially the complete response rate, are very promising but they need to be validated in larger cohorts.
An alternative strategy to improve the efficacy of ICIs is to combine them with loco-regional therapies (LRTs) such as percutaneous ablation, transarterial chemoembolization (TACE), or radiation therapy44. All these procedures lead to tumor cell death, which releases TAA and pro-inflammatory mediators, which in turn may induce tumor-infiltrating immune cell activation and synergize with ICIs45. Interestingly, early results suggest promising efficacy: in a recent study with 110 patients receiving atezolizumab plus bevacizumab as first-line treatment for unresectable intermediate-stage HCC, alone or in combination with LRTs, among the 38 patients achieving complete response, 35 had received ICIs together with LRTs and only 3 ICIs alone46. Another approach to improve ICIs efficacy uses irreversible electroporation following tumor resection to trigger an immune response in HCC. In several studies, it was shown that this technique induces a protective immune response, which could synergize with ICIs47,48. Many combinations involving LRTs and ICIs are under clinical trial44 but preliminary results highlight the complexity of accurately assessing the clinical benefits and potential harms of such therapeutic combination, as shown in the Imbrave 050 trial49. The extent to which neoadjuvant ICI applied before curative procedures may improve outcomes is also currently tested in clinical trials50.
Overall, numerous therapeutic approaches have been tested in combination with ICIs. As an example, in a recent phase II study, we tested a strategy using an antibody targeting phosphatidylserine in combination with pembrolizumab in HCC patients51. Phosphatidylserine is a cytoplasmic-facing phospholipid with an important role in the regulation of inflammation and immune escape. Our data suggest a synergistic effects with PD-1 blockade, which needs to be further confirmed.
Apart from these strategies based on a combination of multiple treatments with ICIs, other approaches try to improve ICI treatment by themselves. First, recent insights pointed out how IgG subclass and posttranslational modifications impact IgG activity, suggesting potential improvements for PD-1, PD-L1, or CTLA-4 antibodies regarding Fc targets52. Notably, different Fc targets of CTLA-4 antibodies play an important role in Tregs inhibition and this could be a way to significantly improve CTLA-4 targeting therapy. Otherwise, a study focused on PD-1 isoforms and identified the Δ42PD-1 as expressed in a subtype of T cells. In different mouse models, they were able to show that antibodies targeting Δ42PD-1 were more efficient than nivolumab53. Alternative strategies are based on the delivery of ICI molecules to the tumor. Nanoparticles are sub-micron-sized structures with a diameter of 150–500 nm54. Liu and colleagues used nanoparticles to co-deliver PD-L1 antibody in addition to a sonodynamic agent (Chlorin E6) in a syngeneic mouse model55. This led to an efficient delivery of the compound and a synergistic effect of the two approaches boosting the effect of PD-L1 antibody. Similarly, peptide-based nanoparticles were used to co-deliver compounds targeting β-catenin and PD-1 simultaneously in syngeneic mouse models56.
CAR T-cell therapy
Chimeric antigen receptor (CAR) T cells represent a revolutionary approach in cancer immunotherapy57. This technology involves genetically engineering a patient’s own T cells to express a CAR that targets a specific antigen present in the tumoral cells. These modified T cells are then expanded in the laboratory and reinfused into the patient, where they can directly recognize and kill cancer cells. Unlike traditional T-cell therapies that rely on the natural ability of T cells to recognize cancer cells, CAR T cells are designed to target tumors with high specificity and potency. In HCC, the application of CAR T-cell therapy holds significant promise. Glypican-3 (GPC3), a protein overexpressed in most HCC cells but not in normal tissues, has been identified as a promising target for CAR T-cell therapy58,59,60. Studies have shown that GPC3-targeted CAR T cells can effectively recognize and eliminate GPC3-positive HCC cells. Several other promising CAR T-cell tumor targets were identified, including alpha-fetoprotein (AFP), Epithelial cell adhesion molecule (EpCAM), Claudin18.2 (CLD18), CD133, and c-MET61. Ongoing clinical trials are still on phase 1 and 2 but some preliminary results are encouraging, notably suggesting a promising anti-tumor activity for CD133-directed CAR T cell therapy in advanced HCC62.
Additional immunotherapies
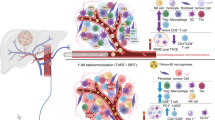
In the past 10 years, the development of novel immunotherapies has been enormously successful, resulting in the identification of novel immune checkpoints such as T-cell immunoglobulin and ITIM domain (TIGIT)63, T-cell immunoglobulin and mucin-domain containing-3 (TIM3)64, lymphocyte activation gene-3 (LAG3)65, CD4766 and B7 homolog 3 protein (B7-H3)67, among others (for a more extensive list, see68). Each of these proteins has distinct ligands and suppress T-cell function through several mechanisms to inhibit T-cell response (Fig. 1). Yet for the moment, phase III clinical trials are missing to evaluate the therapeutic potential of these new targets in HCC patients (Table 1).
This schematic illustrates various immune checkpoint inhibitors (ICIs) and their respective targets within the tumor microenvironment. ICIs include various anti-CTLA-4, anti-PD-1 targeting PD-1 receptors, and anti-PD-L1 antibodies. Magrolimab is depicted targeting CD47 to prevent the ‘don’t eat me’ signal on cancer cells, while TIGIT inhibitors interact with TIGIT receptors on T cells. Other ICIs, such as Enoblituzumab and Orlotamab, are shown to target unspecified antigens, Relatlimab binds LAG3 on T cells, and Cobolimab targets TIM3. The figure underscores the complex interplay between the immune system and cancer cells and highlights the multiplicity of potential therapeutic targets for ICIs.
Hypothetical mechanisms and immune cells involved in resistance to checkpoint inhibitors
The advent of ICIs has marked a significant breakthrough in the treatment of various malignancies, with substantial clinical efficacy across several cancer types69. Particularly in Hodgkin’s disease and desmoplastic melanoma, immune checkpoint inhibitor therapy has achieved outstanding success, with ORR > 70% with the nivolumab plus pembrolizumab combination; but for most cancers (including HCC), treatment responses remain unsatisfactory. Moreover, ~10% of HCC patients under ICIs experience faster and more aggressive tumor progression than expected (known as hyperprogressive disease) for which the mechanisms of action are poorly understood70,71. Resistance to immunotherapy can occur in two different ways. Either patients are primary non-responders, or resistance is acquired after a period of documented response to therapy72. Of note, challenges remain in defining responders and non-responders, given the heterogeneity in patterns of response to ICI, such as spatial or temporal heterogeneity, manifesting within a given patient as mixed responses73.
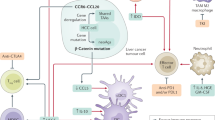
While the exact mechanisms of innate and/or acquired resistance to ICI remain to be fully unveiled, several mechanisms have been hypothesized based on the known mechanisms of action of these therapies and preclinical studies74. These include: decreased neoantigen expression, impaired antigen recognition, ineffective antigen presentation, insufficient priming and activation of tumor-specific T cells, inadequate expansion of T cells or lack of co-stimulation, poor trafficking of the activated effector T cells to the tumor site, insufficient cancer cell recognition by T cells, presence of T-cell inhibitory factors or other T-cell inhibitory immune cells in the tumor microenvironment (TME) (Fig. 2)75. In HCC, the liver’s unique immune microenvironment, characterized by a high prevalence of immunosuppressive cells like Kupffer cells and hepatic stellate cells, also contributes to resistance by creating an immunotolerant environment that diminishes the efficacy of ICIs76. The mechanisms at stake in ICI resistance are complex and involve the crosstalk between many cell populations and subpopulations within the TME.
Illustration of the various mechanisms through which HCC cells may develop resistance to immune checkpoint inhibitors (ICIs). 1) Mutation or altered expression of antigen-presenting molecules on tumor cells leading to reduced T-cell recognition. 2) Impaired antigen presentation. 3) Alterations in the tumor microenvironment, or secretion of immunosuppressive cytokines by tumor cells. 4) Impaired T-cell phenotype, or a physical barrier repressing T-cell infiltration. 5) Altered tumor recognition by T cells. 6) Recruitment of immunosuppressive macrophages, myeloid-derived suppressor cells (MDSCs), or regulatory T cells (Tregs) to the tumor microenvironment, suppressing the activation and proliferation of T cells. 7) Upregulation of alternate immune checkpoints in tumor cells, rendering standard ICIs ineffective. 8) T-cell exhaustion is characterized by the overexpression of multiple inhibitory receptors, diminishing the immune response. The figure underscores the complexity of the tumor-immune microenvironment and the multifaceted nature of immune resistance in HCC, highlighting the need for multi-targeted approaches.
One of the mediators of this process is tumor-associated macrophages (TAMs). In theory, macrophages are dichotomized into M1 and M2 phenotypes, with M1 macrophages exerting pro-inflammatory effects, while M2 macrophages contribute to immunosuppression and tissue repair. However, these subclasses have been difficult to identify in vivo and single-cell RNAseq analysis identified a more complex pattern of distribution of TAMs77. Yet the prevalence of immunosuppressive “M2-like” macrophages, characterized by markers CD163 and CD206, is associated with an aggressive HCC phenotype, advanced tumor stage, and poor survival outcomes78. The presence of these immunosuppressive TAMs, especially at the tumor margin, is linked to adverse clinical features like vascular invasion, tumor multiplicity, and fibrous capsule formation79. Even if the role of the TAMs in ICI resistance has not been fully established in HCC, TAMs remain on the top of the suspects list. Regarding the potential mechanisms, studies in other cancers suggest that “M2-like” macrophages inhibit cytotoxic T cell through IL-10 secretion, and promote immunosuppressive phenotype in other macrophages, NK cells, and Tregs by IL-6, VEGF, and CSF-180,81,82.
Tregs are another cell population with a crucial role in immune exhaustion and resistance to ICIs. Naturally occurring FoxP3 + CD25 + CTLA-4 + CD4+ Tregs are indispensable for immunological self-tolerance. They come from the thymus and derive from the differentiation of T cells with intermediate T-cell receptor (TCR) affinity for self-peptide/MHC ligands while T cells with low TCR affinity differentiate into naive conventional T cells17,83. Tregs are thus able to recognize self-antigens, which may also be tumor-associated neoantigens. In addition to these thymus-derived Tregs, different sets of FoxP3 positive or negative immunosuppressive Tregs can differentiate in the tumor tissue from conventional T cells; the mechanisms at stake are not fully understood but involve IL-2 and TGF-β84. In general, high Treg/CD8 + T-cell ratios in tumors correlate with tumor progression and poor survival85,86. Tregs inhibit antigen presentation on APCs via CTLA-4 and have other immunosuppressive functions through the cell surface molecules, CD25, CD39, and CD73, and the cytokines IL-10, IL-35, and TGF-β17. PD-1 also inhibits Tregs activity as PD-1 blockade can result in increased Tregs activation87. PD-1 positive Tregs in tumors may undermine PD-1 blockade immunotherapy, as shown by the positive correlation between PD-1 positive Treg and hyperprogressive disease in gastric cancer88.
In addition to TAMs and Tregs, tumor-associated neutrophils (TANs) can also be immunosuppressive. Under activation by cancer-associated fibroblasts (CAFs) or HCC cells, they can express PD-L1 and release anti-inflammatory molecules, such as IL8, TNF, and CCl2, which inhibits T-cell activation89, or promotes Tregs recruitment90. Myeloid-derived suppressor cells (MDSCs) are a heterogeneous subset of myeloid cells that have been shown to inhibit T-cell responses in cancer and HCC. MDSCs can inhibit CD8 + T-cell proliferation and their accumulation is associated with poor survival rates in HCC patients91. More, MDSCs can promote Tregs and repress NK cell cytotoxicity by NKp30-dependent cell contact92,93.
Mechanisms of resistance to checkpoint inhibitors: learning from patients
In addition to cell-based studies to characterize the mechanisms of resistance to ICIs, an alternative strategy is to analyze tumor samples to compare the molecular, genetic, and clinical features of patient responders with non-responders. In an elegant study by Zhu and colleagues, tumor samples from 358 HCC patients treated with atezolizumab plus bevacizumab were enrolled from the GO30140 phase 1b and IMbrave 150 phase 3 trials94. The authors discovered that pre-existing immunity, marked by dense intratumoural CD8 + T cells, was linked to improved clinical outcomes. Conversely, reduced benefits were associated with a high ratio of Tregs to effector T cells and the expression of oncofetal genes, as well as with β-catenin mutation. The study also indicated that improved outcomes from the combination therapy, as opposed to atezolizumab alone, were linked to high expression of VEGF Receptor 2, Tregs, and myeloid inflammation signatures. These findings, validated through analyses of pre- and post-treatment biopsies, in situ analyses, and in vivo mouse models, suggest that the anti-VEGF component might synergize with anti-PD-L1 therapy by targeting angiogenesis, Tregs proliferation, and myeloid cell inflammation. The study also analyzed the potential effect of the tumor mutational burden (TMB) on the response to ICIs and showed some inconclusive results. In theory, elevated TMB, often caused by DNA repair deficiency, leads to high level of neoantigens, which increases the immune response and the capacity to target ICIs95,96. However, a recent study in lung and colon cancer showed that high mutational burden can at the same time leads to strong tumor heterogeneity, which jeopardizes the efficacy of ICIs97. In turn, TMB are insufficient to predict response to ICIs and the presence of clonal neoantigens appears to be a better alternative. Overall, the study by Zhu and colleagues confirmed the potential of analyzing the association between treatment response and the patient molecular profiles. Their study also identified a gene signature predicting progression-free survival after atezolizumab-bevacizumab initiation (called the atezolizumab-bevacizumab response signature). Later, this signature was validated in some independent cohorts by Zeng and colleagues and the signature was even predicted based on the analysis of patient pathology slides using artificial intelligence-based algorithms98. This technic could be used in the future to predict ICIs outcome in patients and better assign future treatments.
Another study from Liu and colleagues performed spatial transcriptomics analysis in 11 HCC samples treated with atezolizumab plus bevacizumab (6 non-responders, 5 responders) and highlighted a histological structure called “immune barrier”, only in non-responders99. This structure, composed of TAMs expressing osteopontin interacting with CAFs could act as a physical barrier to T-cell infiltration. Osteopontin is highly expressed in HCC cells and is a well-established predictor of tumor progression and poor outcome100,101. Osteopontin secretion by tumoural cells activates macrophages migration through CD44 signaling102; these activated macrophages further secrete CSF-1, which eventually induces PD-L1 expression in HCC cells82. Moreover, single-cell analysis of HCC patients identified ostepontin as a marker of a subpopulation of cancer-specific macrophages103, which is consistent with the data from Liu and colleagues on a potential immune barrier in HCC99. The concept of the immune barrier can be linked with the concept of “fibrous nest”104, or “fibrotic niche”, an histological structure observed in a subset of HCC patients, that we recently characterized by matrisome analysis105. We showed that this phenotype is associated with cancer-specific extracellular matrix remodeling, signatures of Wnt and TGFβ signaling, and immune evasion. All this is consistent with the immune classification of HCC, initiated by Sia and recently refined by Montironi and colleagues106,107. For long HCCs have been classified based on transcriptomic profile, and works from different teams yields to different classifications108,109,110,111,112,113, ultimately integrated into a system where HCC are divided into two groups: proliferative, associated with poor outcome and TP53 mutation and non-proliferative, associated with better outcome and β-catenin (CTNNB1) mutation. In the immune-based classification, patients are first divided into two groups: inflamed and non-inflamed, based on the expression of a series of markers of immune activation. Within the inflamed group, patients are subdivided into immune-active, immune-exhausted, and immune-like. Patients expressing the Hoshida’s S1/Wnt/TGFβ signature111 are found mostly within the immune active and immune exhausted groups, confirming the link between activation of Wnt/β-Catenin and TGF-β signaling pathways with immune evasion. Both Wnt and TGF-β signaling are known to promote an immunosuppressive TME in HCC. Wnt signaling leads to T-cell exclusion and resistance to ICI therapy through a decrease in chemokines secretion114. Elevated TGF-β signaling increase PD-L1 expression and stimulate Tregs expansion, thus disrupting the effectiveness of both anti-PD-L1 and anti-CTLA-4 antibodies115,116,117. In other cancer types, the decrease in TGF-β-induced collagen deposition has been shown to reactivate adaptative immune response and to improve the efficacy of ICIs, confirming the link between intratumour fibrosis and resistance to immunotherapy118,119,120. Overall, it is likely that TGF-β secretion by tumor cells in immune exhausted patients induce an immunosuppressive TME and activate CAFs, which form a collagen-based fibrous nest, acting as an immune barrier for infiltrating T cells.
Within the non-inflamed group, patients are subdivided into intermediate and immune-excluded. Patients with CTNNB1 mutation, which represent ~30% of HCCs5, are mostly associated with the immune-excluded subclass, consistent with their known resistance to ICIs121,122. Even if the mechanisms of immune exclusion driven by CTNNB1 mutation are not fully understood, a model based on hydrodynamic tail vein injection (HDTV) of a mutated CTNNB1 showed that CTNNB1 mutation impairs the recruitment of DCs and subsequent T-cell activation, mediated by a reduction in CCL5122. Another study showed that the immunosuppressive phenotype in CTNNB1 mutated HCC was mediated by TNFRSF19-mediated repression of cytokines secretion123. Of note, HCC with CTNNB1 mutation was not resistant to ICIs in an HDTV-based mouse model94. Overall, the association between the immune-based molecular classification and response to immunotherapy is appealing but needs to be confirmed in large prospective patient cohorts. This could lead to improving our comprehension of the molecular mechanisms of resistances in different patient subsets and to the rise of personalized medicine in advanced HCCs.
In addition to these approaches based on patient clustering, alternative signaling pathways activated in HCC have been shown to have some immunomodulatory features and appear as interesting therapeutic targets. The EGFR‐P38 MAPK axis in HCC cells also enhances immunosuppression by upregulating PD‐L1 expression and suppressing HLA‐I expression124. The loss of PTEN, a tumor suppressor gene frequently mutated in HCC125, can activate the PI3K signaling pathway, leading to decreased T-cell infiltration and increased immunosuppression. This loss impairs the stimulation of pathways like type I interferon and NF-κB, contributing to tumor progression due to the immunosuppressive TME126,127. All these proteins identified as immunomodulators could be targeted, which could improve the efficacy of ICIs in HCC.
Combination therapy: learning from preclinical models
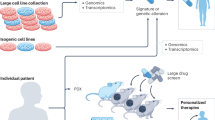
The advancement of ICI therapy in cancer has been greatly facilitated by the recent evolution of preclinical models. Traditionally, the study of liver cancer relied on cell-based models and in vivo xenografts in immunodeficient mice. These models, while invaluable in understanding cancer biology and evaluating the therapeutic potential of chemotherapies, fell short in elucidating the complex interactions between tumor cells and the immune system, which is critical to assessing ICI efficacy. The growing need to accurately assess the potential of ICIs necessitated a shift toward models that embody the intricacies of the tumor-immune microenvironment. Coculture systems mark a step forward, allowing direct interactions between tumor cells and some immune cells in a controlled environment. In theory, they could offer insights into the immunomodulatory effects of ICIs, but these systems are limited by their lack of architectural complexity and the absence of a full physiological immune system. Patient-derived organoids, represent a more physiologically relevant model, preserving the three-dimensional architecture and cellular heterogeneity of tumors128. They serve as a bridge between traditional cell culture and in vivo studies, providing a more accurate platform for drug screening. Their limitation is the absence of a competent immune system, which can be bypassed by combining coculture systems and organoids129,130. Another alternative is the use of patient-derived spheroids, cultured in patients-derived serum131. This system provides all the advantages of the previous one but displays a complete immune system, which can be used to assess the effects of ICIs. Using such a system, we discovered a previously undiscovered immunomodulatory capacity of a therapy targeting Claudin-1, a protein expressed on the cell surface of HCC cells. Treatment with CLDN1-specific antibodies has been shown to markedly inhibit HCC growth by inhibiting pro-carcinogenic signaling and reprogramming the TME132. Syngeneic mouse models, wherein tumor cells from a mouse strain are implanted into a genetically identical host, preserve an intact immune system, thus providing an invaluable context for studying ICIs133. They offer insights into the antitumour immune response and the development of resistance. However, these models often lack the genetic diversity and complexity of human HCC, potentially oversimplifying the immune TME. Chemically induced HCC models in mice replicate the multistage development of liver cancer, providing a spectrum of diseases from dysplasia to carcinoma134,135,136. They are valuable for studying the natural evolution of HCC and the immune responses at different stages. Yet, the long latency and the variability in tumor development are significant drawbacks, posing challenges for timely and uniform study designs. Diethylnitrosamine, in particular, the most widely used model of chemically induced HCC, apart from its long time to tumor development (8–12 months), generates tumors with genetic mutations that poorly recapitulate the mutational landscape of human HCC137 and harboring poor immune infiltration138. This model is often associated with chemically induced or diet-induced liver fibrosis, either with carbon tetrachloride or Western diet in order to accelerate the tumor development and to get closer to the physiopathology of HCC development134,139. Similarly, genetically engineered mouse models with transgenic overexpression of an oncogene and/or ablation of an anti-oncogene, offer the possibility to evaluate the effect of ICIs in immunocompetent animal, but by definition, they can only recapitulate a narrow spectrum of genetic alterations, which can be problematic for the translation to human140. The HDTV technique is a relatively recent innovation, enabling the study of gene function in liver carcinogenesis through the rapid introduction of genetic material into hepatocytes141. This method can mimic the genetic alterations seen in human HCC and allows for the study of tumor-immune interactions in an intact immune system. By the choice of the oncogenes overexpression, or the anti-oncogenes deletion, they also provide a variety of cancer phenotypes, associated with different immune profiles, which mimics, to a certain extend, the variability of human HCC142,143. More, models using some plasmid combinations of oncogenes are sensitive to ICIs, while others are resistant143,144. Humanized mice, engineered to possess a human immune system, provide a critical platform for evaluating the efficacy of ICIs in a context that closely mimics human immune responses145. They are particularly useful for studying ICIs targeting human-specific antigens. The cons include the high cost, the need for specialized facilities, and the fact that the reconstituted human immune system may not fully recapitulate the diversity and functionality of its natural counterpart.
Table 2 provide an extended list of the preclinical studies that used immunocompetent mouse models to study the possibility of combination therapy of ICIs with other drugs. They were performed using a variety of mouse models including syngeneic, HDTV, chemically induced, or transgenic mouse models. The most widely used model was the subcutaneous injection of Hepa1-6 cells. This HCC mouse cell line originates from the BW7756 cells, generated by Jackson laboratory from C57L/J mice in the 60’s and first used in research in 1971146, before it was in vitro subcloned into different Hepa1 variants147 and that the Hepa1-6 was identified148. Since then, the Hepa1-6 cell line has emerged as a reliable tool to easily and cost-effectively generate fast-growing tumors, both in C57L and C57/BL6, upon subcutaneous or orthotopic (i.e., intrahepatic) injection. Tumors from this model partly respond to ICIs but are sensitive to a broad spectrum of combination therapies, which improve the efficacy of ICIs. Recently, Zabransky and colleagues performed cytometry by time of flight to profile the TME of different syngeneic mouse models of HCC, including Hepa1-6149. They highlighted a rather important CD8 T-cell infiltration as well as PD-L1 expression in Hepa1-6 orthotopic model. They also performed integration with human data and found only a few HCC samples with immune profiles matching the one of Hepa1-6, which questions the translationality of data using this model. Of note, while Hepa1-6 subcutaneously injected were sensitive to anti-PD-1 therapy, it was not the case for orthotopic injection in this study, underlying the importance of orthotopic models to better recapitulate the characteristics of the liver TME for ICIs evaluation.
As shown in Table 2, a broad spectrum of therapeutic targets has shown a potential to improve the efficacy of ICIs, which underlines the wide possibilities for refining current treatments. First, some studies have been using ICIs in combination with standard chemotherapy such as oxaliplatin, or with multikinase inhibitors already approved as single agents for the treatment of HCC (sorafenib or Lenvatinib). These studies suggest some potential in combining ICIs with standard treatments, as recently performed for the treatment of cholangiocarcinoma, where ICIs have been approved in combination with chemotherapy12,150. Alternatively, studies tried some molecules from traditional medicine like Scutellarin151 or YIV-906152, which highlight the potential of drugs already known to have anticancer properties to improve ICIs-based therapy. Studies like the one from Bao and colleagues also confirm that vascular normalization strategies can enhance ICI efficacy153. By a combination of VEGF/VEGFR2 inhibitors with anti-PD-1 therapy, they showed a significant increase in CD8 T-cell infiltration, a significant reduction in tumor size, and improved survival rates in treated mice. Another study used regorafenib, a multikinase inhibitor known for its anti-angiogenic properties, with PD-1 blockade and found that regorafenib not only hindered angiogenesis but also altered the TME to make it more receptive to ICIs154. As expected, a majority of studies showing improvement of ICIs used immunomodulatory agents, like antibodies targeting TIGIT144, CXCR2134, CSF-1-R82,155, osteopontin99 or triggering receptor expressed on myeloid cells-1 (TREM-1)156. Others used nonsteroidal anti-inflammatory drugs (meloxicam)157 or interferon-α158,159, or approaches closer to a vaccination strategy, by the use of components triggering an immunization in the mouse, by using polyinosinic-polycytidylic acid160, DCs vaccine161 or AFP immunization162. Another strategy is to focus on the metabolic pathways associated with HCC and highlight that modulating these networks has the potential to improve immunotherapy. For example, Luo and colleagues identified Prmt5, encoding protein arginine N-methyltransferase 5, as a key metabolic modulator of MYC-induced HCC; and by disrupting this pathway they could enhance the response to anti-PD-1 therapy163. Other drugs targeting liver metabolisms showed some potential, like cholecystokinina antagonist164 or peroxisome proliferator-activated receptor gamma antagonist165. More analysis focused on novel molecular and genetic targets with unknown roles in liver cancer, such as N6-methyladenosine reader YTHDF1, Poly(ADP-ribose) glycohydrolase, or Cholecystokinin-B Receptor. These three new therapeutic targets were shown to be targetable and to synergize with ICIs164,166,167. Collectively, all these studies demonstrate the potential of combining ICIs with a variety of agents targeting different aspects of tumor biology and the TME. Each approach offers unique insights into enhancing the efficacy of immunotherapy in HCC, emphasizing the need for multifaceted and tailored treatment strategies to overcome the complex nature of the disease.
Conclusion and future avenues
Emerging strategies in the treatment of HCC through ICIs reveal the multifaceted nature of cancer therapy. The development and utilization of immunocompetent preclinical models are instrumental in understanding the complex interactions between tumor cells and the immune system, but significant challenges arise when translating these findings into clinical practice. Syngeneic mouse models, genetically engineered mice, chemically induced, and diet-induced models only partially capture the diversity of the tumor-immune microenvironment from human HCC. Bridging the gap between preclinical research and clinical application remains a significant challenge in cancer research. Nevertheless, the exploitation of combination therapies using these models targeting the TME from multiple angles has revealed important new information to improve cancer therapy. Investigating the molecular diversity of HCC has uncovered new therapeutic targets. The potential of CAR T-cell therapy remains still to be determined for HCC but may open a perspective for a more personalized treatment approach. As we move forward, the integration of these diverse therapeutic strategies promises to enhance the efficacy of current therapies. The combination of discovery and innovation will bring the field closer to more effective, personalized treatments for HCC patients—a key unmet medical need. The collective effort of clinicians and scientists will undoubtedly pave the way for improved efficacy and safety and, ultimately, improved prognosis for patients.
Data availability
No datasets were generated or analyzed during the current study.
Abbreviations
- APCs:
-
antigen-presenting cells
- B7-H3:
-
B7 homolog 3 protein
- BCLC:
-
Barcelona clinic liver cancer
- CAFs:
-
cancer-associated fibroblasts
- CAR:
-
Chimeric antigen receptor
- CTLA-4:
-
cytotoxic T-lymphocyte-associated protein 4
- DCs:
-
dendritic cells
- HCC:
-
Hepatocellular carcinoma
- HDTV:
-
hydrodynamic tail vein injection
- ICIs:
-
immune checkpoints inhibitors
- ITSM:
-
immune receptor tyrosine-based switch motif
- ITIM:
-
immune receptor tyrosine-based inhibitory motif
- LAG3:
-
lymphocyte activation gene-3
- LRTs:
-
loco-regional therapies
- MDSCs:
-
myeloid-derived suppressor cells
- MHC:
-
major histocompatibility complex
- mOS:
-
median overall survival
- NF-κB:
-
nuclear factor kappa-light-chain-enhancer of activated B cells
- ORR:
-
objective response rate
- PD-L1:
-
programmed cell death ligand 1
- TAA:
-
Tumor-associated antigens
- TACE:
-
transarterial chemoembolization
- TAMs:
-
tumor-associated macrophages
- TANs:
-
tumor-associated neutrophils
- TCR:
-
T-cell receptor
- TIGIT:
-
T-cell immunoglobulin and ITIM domain
- TIM3:
-
T-cell immunoglobulin and mucin-domain containing-3
- Tregs:
-
regulatory T cells
- TME:
-
tumor microenvironment
- TMB:
-
tumor mutational burden
References
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Rumgay, H. et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J. Hepatol. 77, 1598–1606 (2022).
Estes, C. et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 69, 896–904 (2018).
Singal, A. G., Kanwal, F. & Llovet, J. M. Global trends in hepatocellular carcinoma epidemiology: implications for screening, prevention and therapy. Nat. Rev. Clin. Oncol. 20, 864–884 (2023).
Llovet, J. M. et al. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 7, 6 (2021).
Sia, D., Villanueva, A., Friedman, S. L. & Llovet, J. M. Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology 152, 745–761 (2017).
Tabrizian, P. et al. Ten-year outcomes of liver transplant and downstaging for hepatocellular carcinoma. JAMA Surg. 157, 779–788 (2022).
Reveron-Thornton, R. F. et al. Global and regional long-term survival following resection for HCC in the recent decade: a meta-analysis of 110 studies. Hepatol. Commun. 6, 1813–1826 (2022).
Llovet, J. M. et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 359, 378–390 (2008).
Singal, A. G. et al. AASLD practice guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology, 78, 1922–1965 (2023).
Abou-Alfa G. K. et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 1, EVIDoa2100070 (2022).
Finn, R. S. et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 382, 1894–1905 (2020).
Cheng, A. L. et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 76, 862–873 (2022).
Kornepati, A. V. R., Vadlamudi, R. K. & Curiel, T. J. Programmed death ligand 1 signals in cancer cells. Nat. Rev. Cancer 22, 174–189 (2022).
Riley, J. L. PD-1 signaling in primary T cells. Immunol. Rev. 229, 114–125 (2009).
Li, Q. et al. Antibody-based cancer immunotherapy by targeting regulatory T cells. Front. Oncol. 13, 1157345 (2023).
Tay, C., Tanaka, A. & Sakaguchi, S. Tumour-infiltrating regulatory T cells as targets of cancer immunotherapy. Cancer Cell 41, 450–465 (2023).
Cheng, X. et al. Structure and interactions of the human programmed cell death 1 receptor. J. Biol. Chem. 288, 11771–11785 (2013).
Gao, Q. et al. Overexpression of PD-L1 significantly associates with tumour aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin. Cancer Res. 15, 971–979 (2009).
Zeng, T. et al. Expression pattern of PD-1/PD-L1 in primary liver cancer with clinical correlation. Liver Int. 43, 1995–2001 (2023).
Sugiura, D. et al. Restriction of PD-1 function by cis-PD-L1/CD80 interactions is required for optimal T cell responses. Science 364, 558–566 (2019).
Li, Q., Han, J., Yang, Y. & Chen, Y. PD-1/PD-L1 checkpoint inhibitors in advanced hepatocellular carcinoma immunotherapy. Front. Immunol. 13, 1070961 (2022).
Wang, J. et al. Clinical outcomes and influencing factors of PD-1/PD-L1 in hepatocellular carcinoma. Oncol. Lett. 21, 279 (2021).
Muhlbauer, M. et al. PD-L1 is induced in hepatocytes by viral infection and by interferon-alpha and -gamma and mediates T cell apoptosis. J. Hepatol. 45, 520–528 (2006).
Brunet, J. F. et al. A new member of the immunoglobulin superfamily-CTLA-4. Nature 328, 267–270 (1987).
Leach, D. R., Krummel, M. F. & Allison, J. P. Enhancement of antitumour immunity by CTLA-4 blockade. Science 271, 1734–1736 (1996).
Rudd, C. E., Taylor, A. & Schneider, H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol. Rev. 229, 12–26 (2009).
Yang, W. et al. A novel CTLA-4 blocking strategy based on nanobody enhances the activity of dendritic cell vaccine-stimulated antitumour cytotoxic T lymphocytes. Cell Death Dis. 14, 406 (2023).
Krummel, M. F. & Allison, J. P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 182, 459–465 (1995).
Hannani, D. et al. Anticancer immunotherapy by CTLA-4 blockade: obligatory contribution of IL-2 receptors and negative prognostic impact of soluble CD25. Cell Res. 25, 208–224 (2015).
Hu, H., Rudd, C. E. & Schneider, H. Src kinases Fyn and Lck facilitate the accumulation of phosphorylated CTLA-4 and its association with PI-3 kinase in intracellular compartments of T-cells. Biochem. Biophys. Res. Commun. 288, 573–578 (2001).
Schneider, H., Smith, X., Liu, H., Bismuth, G. & Rudd, C. E. CTLA-4 disrupts ZAP70 microcluster formation with reduced T cell/APC dwell times and calcium mobilization. Eur. J. Immunol. 38, 40–47 (2008).
Kim, G. R. & Choi, J. M. Current understanding of cytotoxic T lymphocyte antigen-4 (CTLA-4) Signaling in T-cell biology and disease therapy. Mol. Cells 45, 513–521 (2022).
Rowshanravan, B., Halliday, N. & Sansom, D. M. CTLA-4: a moving target in immunotherapy. Blood 131, 58–67 (2018).
Han, Y. et al. Human CD14+ CTLA-4+ regulatory dendritic cells suppress T-cell response by cytotoxic T-lymphocyte antigen-4-dependent IL-10 and indoleamine-2,3-dioxygenase production in hepatocellular carcinoma. Hepatology 59, 567–579 (2014).
Kalathil, S., Lugade, A. A., Miller, A., Iyer, R. & Thanavala, Y. Higher frequencies of GARP(+)CTLA-4(+)Foxp3(+) T regulatory cells and myeloid-derived suppressor cells in hepatocellular carcinoma patients are associated with impaired T-cell functionality. Cancer Res. 73, 2435–2444 (2013).
Pedroza-Gonzalez, A. et al. GITR engagement in combination with CTLA-4 blockade completely abrogates immunosuppression mediated by human liver tumour-derived regulatory T cells ex vivo. Oncoimmunology 4, e1051297 (2015).
Sangro, B. et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J. Hepatol. 59, 81–88 (2013).
Yau, T. et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 6, e204564 (2020).
Sové R. J. et al. Virtual clinical trials of anti-PD-1 and anti-CTLA-4 immunotherapy in advanced hepatocellular carcinoma using a quantitative systems pharmacology model. J. Immunother. Cancer. 10, e005414 (2022).
Hu, Z., Guo, X., Li, Z., Meng, Z. & Huang, S. The neoantigens derived from transposable elements - a hidden treasure for cancer immunotherapy. Biochim. Biophys. Acta Rev. Cancer 1879, 189126 (2024).
Guasp, P., Reiche, C., Sethna, Z. & Balachandran, V. P. RNA vaccines for cancer: principles to practice. Cancer Cell 42, 1163–1184 (2024).
Yarchoan, M. et al. Personalized neoantigen vaccine and pembrolizumab in advanced hepatocellular carcinoma: a phase 1/2 trial. Nat. Med. 30, 1044–1053 (2024).
Llovet, J. M. et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 18, 293–313 (2021).
McLaughlin, M. et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat. Rev. Cancer 20, 203–217 (2020).
Kudo, M. et al. Achievement of complete response and drug-free status by atezolizumab plus bevacizumab combined with or without curative conversion in patients with transarterial chemoembolization-unsuitable, intermediate-stage hepatocellular carcinoma: a multicenter proof-of-concept study. Liver Cancer 12, 321–338 (2023).
Dai, Z. et al. Irreversible electroporation induces CD8(+) T cell immune response against post-ablation hepatocellular carcinoma growth. Cancer Lett. 503, 1–10 (2021).
Qian, J. et al. Blocking exposed PD-L1 elicited by nanosecond pulsed electric field reverses dysfunction of CD8(+) T cells in liver cancer. Cancer Lett. 495, 1–11 (2020).
Qin, S. et al. Atezolizumab plus bevacizumab versus active surveillance in patients with resected or ablated high-risk hepatocellular carcinoma (IMbrave050): a randomised, open-label, multicentre, phase 3 trial. Lancet 402, 1835–1847 (2023).
Llovet, J. M. et al. Adjuvant and neoadjuvant immunotherapies in hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 21, 294–311 (2024).
Hsiehchen, D. et al. The phosphatidylserine targeting antibody bavituximab plus pembrolizumab in unresectable hepatocellular carcinoma: a phase 2 trial. Nat. Commun. 15, 2178 (2024).
Nimmerjahn, F., Vidarsson, G. & Cragg, M. S. Effect of posttranslational modifications and subclass on IgG activity: from immunity to immunotherapy. Nat. Immunol. 24, 1244–1255 (2023).
Tan, Z. et al. Isoformic PD-1-mediated immunosuppression underlies resistance to PD-1 blockade in hepatocellular carcinoma patients. Gut 72, 1568–1580 (2023).
Pavelic, K. et al. Nanoparticles in medicine: current status in cancer treatment. Int J. Mol. Sci. 24, 12827 (2023).
Liu, Y. et al. Nanobubble-based anti-hepatocellular carcinoma therapy combining immune check inhibitors and sonodynamic therapy. Nanoscale Adv. 4, 4847–4862 (2022).
Zhou, Z. et al. Targeting beta-catenin and PD-L1 simultaneously by a racemic supramolecular peptide for the potent immunotherapy of hepatocellular carcinoma. Theranostics 13, 3371–3386 (2023).
Labanieh, L. & Mackall, C. L. CAR immune cells: design principles, resistance and the next generation. Nature 614, 635–648 (2023).
Jiang, Z. et al. Anti-GPC3-CAR T cells suppress the growth of tumour cells in patient-derived xenografts of hepatocellular carcinoma. Front. Immunol. 7, 690 (2016).
Wu, X. et al. Combined antitumour effects of sorafenib and GPC3-CAR T cells in mouse models of hepatocellular carcinoma. Mol. Ther. 27, 1483–1494 (2019).
Li, D. et al. Persistent polyfunctional chimeric antigen receptor T cells that target glypican 3 eliminate orthotopic hepatocellular carcinomas in mice. Gastroenterology 158, 2250–2265.e2220 (2020).
Ozer, M., Goksu, S. Y., Akagunduz, B., George, A. & Sahin, I. Adoptive cell therapy in hepatocellular carcinoma: a review of clinical trials. Cancers 15, 1808 (2023).
Dai, H. R. et al. Efficacy and biomarker analysis of CD133-directed CAR T cells in advanced hepatocellular carcinoma: a single-arm, open-label, phase II trial. Oncoimmunology 9, 1846926 (2020).
Jantz-Naeem, N. et al. TIGIT signaling and its influence on T cell metabolism and immune cell function in the tumour microenvironment. Front. Oncol. 13, 1060112 (2023).
Tian, T. & Li, Z. Targeting Tim-3 in cancer with resistance to PD-1/PD-L1 blockade. Front. Oncol. 11, 731175 (2021).
Huo, J. L., Wang, Y. T., Fu, W. J., Lu, N. & Liu, Z. S. The promising immune checkpoint LAG-3 in cancer immunotherapy: from basic research to clinical application. Front. Immunol. 13, 956090 (2022).
Son, J. et al. Inhibition of the CD47-SIRPalpha axis for cancer therapy: a systematic review and meta-analysis of emerging clinical data. Front. Immunol. 13, 1027235 (2022).
Zhou, W. T. & Jin, W. L. B7-H3/CD276: an emerging cancer immunotherapy. Front. Immunol. 12, 701006 (2021).
Dutta, S., Ganguly, A., Chatterjee, K., Spada, S. & Mukherjee, S. Targets of immune escape mechanisms in cancer: basis for development and evolution of cancer immune checkpoint inhibitors. Biology 12, 218 (2023).
Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018).
Pinter, M., Scheiner, B. & Pinato, D. J. Immune checkpoint inhibitors in hepatocellular carcinoma: emerging challenges in clinical practice. Lancet Gastroenterol. Hepatol. 8, 760–770 (2023).
Wei, Z. & Zhang, Y. Immune cells in hyperprogressive disease under immune checkpoint-based immunotherapy. Cells 11, 1758 (2022).
Sharma, P., Hu-Lieskovan, S., Wargo, J. A. & Ribas, A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 168, 707–723 (2017).
Michielin, O., Lalani, A. K., Robert, C., Sharma, P. & Peters, S. Defining unique clinical hallmarks for immune checkpoint inhibitor-based therapies. J. Immunother. Cancer 10, e003024 (2022).
Jenkins, R. W., Barbie, D. A. & Flaherty, K. T. Mechanisms of resistance to immune checkpoint inhibitors. Br. J. Cancer 118, 9–16 (2018).
Xie, Q., Zhang, P., Wang, Y., Mei, W. & Zeng, C. Overcoming resistance to immune checkpoint inhibitors in hepatocellular carcinoma: challenges and opportunities. Front. Oncol. 12, 958720 (2022).
Ringelhan, M., Pfister, D., O’Connor, T., Pikarsky, E. & Heikenwalder, M. The immunology of hepatocellular carcinoma. Nat. Immunol. 19, 222–232 (2018).
Roszer, T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediat. Inflamm. 2015, 816460 (2015).
Dong, P. et al. CD86(+)/CD206(+), diametrically polarized tumour-associated macrophages, predict hepatocellular carcinoma patient prognosis. Int J. Mol. Sci. 17, 320 (2016).
Ding, T. et al. High tumour-infiltrating macrophage density predicts poor prognosis in patients with primary hepatocellular carcinoma after resection. Hum. Pathol. 40, 381–389 (2009).
Donne, R. & Lujambio, A. The liver cancer immune microenvironment: therapeutic implications for hepatocellular carcinoma. Hepatology 77, 1773–1796 (2023).
Cassetta, L. & Pollard, J. W. A timeline of tumour-associated macrophage biology. Nat. Rev. Cancer 23, 238–257 (2023).
Zhu, Y. et al. Disruption of tumour-associated macrophage trafficking by the osteopontin-induced colony-stimulating factor-1 signalling sensitises hepatocellular carcinoma to anti-PD-L1 blockade. Gut 68, 1653–1666 (2019).
Klein, L., Kyewski, B., Allen, P. M. & Hogquist, K. A. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see). Nat. Rev. Immunol. 14, 377–391 (2014).
Bayati, F. et al. The therapeutic potential of regulatory T cells: challenges and opportunities. Front. Immunol. 11, 585819 (2020).
Tu, J. F. et al. Regulatory T cells, especially ICOS(+) FOXP3(+) regulatory T cells, are increased in the hepatocellular carcinoma microenvironment and predict reduced survival. Sci. Rep. 6, 35056 (2016).
Schoenberg, M. B. et al. The predictive value of tumour infiltrating leukocytes in hepatocellular carcinoma: a systematic review and meta-analysis. Eur. J. Surg. Oncol. 47, 2561–2570 (2021).
Vick, S. C., Kolupaev, O. V., Perou, C. M. & Serody, J. S. Anti-PD-1 checkpoint therapy can promote the function and survival of regulatory T cells. J. Immunol. 207, 2598–2607 (2021).
Kamada, T. et al. PD-1(+) regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc. Natl. Acad. Sci. USA 116, 9999–10008 (2019).
Cheng, Y. et al. Cancer-associated fibroblasts induce PDL1+ neutrophils through the IL6-STAT3 pathway that foster immune suppression in hepatocellular carcinoma. Cell Death Dis. 9, 422 (2018).
Zhou, S. L. et al. Tumour-associated neutrophils recruit macrophages and t-regulatory cells to promote progression of hepatocellular carcinoma and resistance to sorafenib. Gastroenterology 150, 1646–1658.e1617 (2016).
Zhou, J. et al. Hepatoma-intrinsic CCRK inhibition diminishes myeloid-derived suppressor cell immunosuppression and enhances immune-checkpoint blockade efficacy. Gut 67, 931–944 (2018).
Hoechst, B. et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology 50, 799–807 (2009).
Hoechst, B. et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology 135, 234–243 (2008).
Zhu, A. X. et al. Molecular correlates of clinical response and resistance to atezolizumab in combination with bevacizumab in advanced hepatocellular carcinoma. Nat. Med. 28, 1599–1611 (2022).
Miao, D. et al. Genomic correlates of response to immune checkpoint blockade in microsatellite-stable solid tumours. Nat. Genet. 50, 1271–1281 (2018).
McGranahan, N. et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 351, 1463–1469 (2016).
Westcott, P. M. K. et al. Mismatch repair deficiency is not sufficient to elicit tumour immunogenicity. Nat. Genet. 55, 1686–1695 (2023).
Zeng, Q. et al. Artificial intelligence-based pathology as a biomarker of sensitivity to atezolizumab-bevacizumab in patients with hepatocellular carcinoma: a multicentre retrospective study. Lancet Oncol. 24, 1411–1422 (2023).
Liu, Y. et al. Identification of a tumour immune barrier in the HCC microenvironment that determines the efficacy of immunotherapy. J. Hepatol. 78, 770–782 (2023).
Song, Z. et al. Osteopontin takes center stage in chronic liver disease. Hepatology 73, 1594–1608 (2021).
Desert, R. et al. Role of hepatocyte-derived osteopontin in liver carcinogenesis. Hepatol. Commun. 6, 692–709 (2022).
Jiang, X. et al. Lipid-injured hepatocytes release sOPN to improve macrophage migration via CD44 engagement and pFak-NFkappaB signaling. Cytokine 142, 155474 (2021).
Sharma, A. et al. Onco-fetal reprogramming of endothelial cells drives immunosuppressive macrophages in hepatocellular carcinoma. Cell 183, 377–394.e321 (2020).
Desert, R. et al. “Fibrous nests” in human hepatocellular carcinoma express a Wnt-induced gene signature associated with poor clinical outcome. Int. J. Biochem. Cell Biol. 81, 195–207 (2016).
Desert, R. et al. Hepatocellular carcinomas, exhibiting intratumour fibrosis, express cancer-specific extracellular matrix remodeling and WNT/TGFB signatures, associated with poor outcome. Hepatology 78, 741–757 (2023).
Sia, D. et al. Identification of an immune-specific class of hepatocellular carcinoma, based on molecular features. Gastroenterology 153, 812–826 (2017).
Montironi, C. et al. Inflamed and non-inflamed classes of HCC: a revised immunogenomic classification. Gut 72, 129–140 (2023).
Lee, J. S. et al. Classification and prediction of survival in hepatocellular carcinoma by gene expression profiling. Hepatology 40, 667–676 (2004).
Boyault, S. et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology 45, 42–52 (2007).
Chiang, D. Y. et al. Focal gains of VEGFA and molecular classification of hepatocellular carcinoma. Cancer Res. 68, 6779–6788 (2008).
Hoshida, Y. et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 69, 7385–7392 (2009).
Desert, R. et al. Human hepatocellular carcinomas with a periportal phenotype have the lowest potential for early recurrence after curative resection. Hepatology 66, 1502–1518 (2017).
Cancer Genome Atlas Research Network. Electronic address wbe, Cancer Genome Atlas Research N. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell 169, 1327–1341.e1323 (2017).
Morita, M. et al. Role of beta-catenin activation in the tumour immune microenvironment and immunotherapy of hepatocellular carcinoma. Cancers 15, 2311 (2023).
David, C. J. & Massague, J. Contextual determinants of TGFbeta action in development, immunity and cancer. Nat. Rev. Mol. Cell Biol. 19, 419–435 (2018).
Flavell, R. A., Sanjabi, S., Wrzesinski, S. H. & Licona-Limon, P. The polarization of immune cells in the tumour environment by TGFbeta. Nat. Rev. Immunol. 10, 554–567 (2010).
Gonzalez-Sanchez, E. et al. The TGF-beta pathway: a pharmacological target in hepatocellular carcinoma? Cancers 13, 3248 (2021).
Li, L. et al. Laminin gamma2-mediating T cell exclusion attenuates response to anti-PD-1 therapy. Sci. Adv. 7, eabc8346 (2021).
Horn, L. A. et al. Remodeling the tumour microenvironment via blockade of LAIR-1 and TGF-beta signaling enables PD-L1-mediated tumour eradication. J. Clin. Invest. 132, e155148 (2022).
Zhang, D. et al. Enhancing CRISPR/Cas gene editing through modulating cellular mechanical properties for cancer therapy. Nat. Nanotechnol. 17, 777–787 (2022).
Harding, J. J. et al. Prospective genotyping of hepatocellular carcinoma: clinical implications of next-generation sequencing for matching patients to targeted and immune therapies. Clin. Cancer Res. 25, 2116–2126 (2019).
Ruiz de Galarreta, M. et al. Beta-catenin activation promotes immune escape and resistance to anti-PD-1 therapy in hepatocellular carcinoma. Cancer Discov. 9, 1124–1141 (2019).
Wong, A. M. et al. Unique molecular characteristics of NAFLD-associated liver cancer accentuate beta-catenin/TNFRSF19-mediated immune evasion. J. Hepatol. 77, 410–423 (2022).
Liu, Z. et al. The EGFR-P38 MAPK axis up-regulates PD-L1 through miR-675-5p and down-regulates HLA-ABC via hexokinase-2 in hepatocellular carcinoma cells. Cancer Commun. 41, 62–78 (2021).
Schulze, K. et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat. Genet. 47, 505–511 (2015).
Vidotto, T. et al. Emerging role of PTEN loss in evasion of the immune response to tumours. Br. J. Cancer 122, 1732–1743 (2020).
Lin, Z. et al. PTEN loss correlates with T cell exclusion across human cancers. BMC Cancer 21, 429 (2021).
LeSavage, B. L., Suhar, R. A., Broguiere, N., Lutolf, M. P. & Heilshorn, S. C. Next-generation cancer organoids. Nat. Mater. 21, 143–159 (2022).
Yuan, J., Li, X. & Yu, S. Cancer organoid co-culture model system: novel approach to guide precision medicine. Front. Immunol. 13, 1061388 (2022).
Zhou Z. et al. Harnessing 3D in vitro systems to model immune responses to solid tumours: a step towards improving and creating personalized immunotherapies. Nat. Rev. Immunol. 24, 18–32 (2023).
Crouchet, E. et al. A human liver cell-based system modeling a clinical prognostic liver signature for therapeutic discovery. Nat. Commun. 12, 5525 (2021).
Roehlen, N. et al. Treatment of HCC with claudin-1-specific antibodies suppresses carcinogenic signaling and reprograms the tumour microenvironment. J. Hepatol. 78, 343–355 (2023).
Blidisel A. et al. Experimental models of hepatocellular carcinoma-a preclinical perspective. Cancers 13, 3651 (2021).
Leslie, J. et al. CXCR2 inhibition enables NASH-HCC immunotherapy. Gut 71, 2093–2106 (2022).
Moeini, A. et al. An immune gene expression signature associated with development of human hepatocellular carcinoma identifies mice that respond to chemopreventive agents. Gastroenterology 157, 1383–1397.e1311 (2019).
Koelsch, N. et al. The crosstalking immune cells network creates a collective function beyond the function of each cellular constituent during the progression of hepatocellular carcinoma. Sci. Rep. 13, 12630 (2023).
Connor, F. et al. Mutational landscape of a chemically-induced mouse model of liver cancer. J. Hepatol. 69, 840–850 (2018).
Liu, K. et al. Novel miRNA-based drug CD5-2 reduces liver tumour growth in diethylnitrosamine-treated mice by normalizing tumour vasculature and altering immune infiltrate. Front. Immunol. 14, 1245708 (2023).
Filliol, A. et al. Opposing roles of hepatic stellate cell subpopulations in hepatocarcinogenesis. Nature 610, 356–365 (2022).
Liu, S. et al. Mouse models of hepatocellular carcinoma: classification, advancement, and application. Front. Oncol. 12, 902820 (2022).
Sebestyen, M. G. et al. Mechanism of plasmid delivery by hydrodynamic tail vein injection. I. Hepatocyte uptake of various molecules. J. Gene Med. 8, 852–873 (2006).
Molina-Sanchez, P. et al. Cooperation between distinct cancer driver genes underlies intertumour heterogeneity in hepatocellular carcinoma. Gastroenterology 159, 2203–2220.e2214 (2020).
Yuen, V. W. et al. Using mouse liver cancer models based on somatic genome editing to predict immune checkpoint inhibitor responses. J. Hepatol. 78, 376–389 (2023).
Chiu, D. K. et al. Hepatocellular carcinoma cells up-regulate PVRL1, stabilizing PVR and inhibiting the cytotoxic T-cell response via TIGIT to mediate tumour resistance to PD1 inhibitors in mice. Gastroenterology 159, 609–623 (2020).
Chuprin, J. et al. Humanized mouse models for immuno-oncology research. Nat. Rev. Clin. Oncol. 20, 192–206 (2023).
Kahan, B. & Levine, L. The occurrence of a serum fetal alpha-1 protein in developing mice and murine hepatomas and teratomas. Cancer Res. 31, 930–936 (1971).
Bernhard, H. P., Darlington, G. J. & Ruddle, F. H. Expression of liver phenotypes in cultured mouse hepatoma cells: synthesis and secretion of serum albumin. Dev. Biol. 35, 83–96 (1973).
Darlington, G. J., Tsai, C. C., Samuelson, L. C., Gumucio, D. L. & Meisler, M. H. Simultaneous expression of salivary and pancreatic amylase genes in cultured mouse hepatoma cells. Mol. Cell Biol. 6, 969–975 (1986).
Zabransky, D. J. et al. Profiling of syngeneic mouse HCC tumour models as a framework to understand anti-PD-1 sensitive tumour microenvironments. Hepatology 77, 1566–1579 (2023).
Kelley, R. K. et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 401, 1853–1865 (2023).
Li, L. et al. Nanodelivery of scutellarin induces immunogenic cell death for treating hepatocellular carcinoma. Int. J. Pharm. 642, 123114 (2023).
Yang, X. et al. YIV-906 potentiated anti-PD1 action against hepatocellular carcinoma by enhancing adaptive and innate immunity in the tumour microenvironment. Sci. Rep. 11, 13482 (2021).
Bao, X. et al. Enhanced anti-PD-1 therapy in hepatocellular carcinoma by tumour vascular disruption and normalization dependent on combretastatin A4 nanoparticles and DC101. Theranostics 11, 5955–5969 (2021).
Shigeta, K. et al. Regorafenib combined with PD1 blockade increases CD8 T-cell infiltration by inducing CXCL10 expression in hepatocellular carcinoma. J. Immunother. Cancer 8, e001435 (2022).
Wei, C. Y. et al. PKCalpha/ZFP64/CSF-1 axis resets the tumour microenvironment and fuels anti-PD1 resistance in hepatocellular carcinoma. J. Hepatol. 77, 163–176 (2022).
Wu, Q. et al. Blocking triggering receptor expressed on myeloid cells-1-positive tumour-associated macrophages induced by hypoxia reverses immunosuppression and anti-programmed cell death ligand 1 resistance in liver cancer. Hepatology 70, 198–214 (2019).
Guangshun, S. et al. Meloxicam inhibits hepatocellular carcinoma progression and enhances the sensitivity of immunotherapy via the MicroRNA-200/PD-L1 pathway. J. Oncol. 2022, 4598573 (2022).
Wang, J. et al. Hepatocellular carcinoma growth retardation and PD-1 blockade therapy potentiation with synthetic high-density lipoprotein. Nano Lett. 19, 5266–5276 (2019).
Zhu, Y. et al. The combination of PD-1 blockade with interferon-alpha has a synergistic effect on hepatocellular carcinoma. Cell Mol. Immunol. 19, 726–737 (2022).
Wen, L. et al. An efficient combination immunotherapy for primary liver cancer by harmonized activation of innate and adaptive immunity in mice. Hepatology 69, 2518–2532 (2019).
Teng, C. F. et al. Combination therapy with dendritic cell vaccine and programmed death ligand 1 immune checkpoint inhibitor for hepatocellular carcinoma in an orthotopic mouse model. Ther. Adv. Med. Oncol. 12, 1758835920922034 (2020).
Lu, X. et al. Combination of AFP vaccine and immune checkpoint inhibitors slows hepatocellular carcinoma progression in preclinical models. J. Clin. Invest. 133, e163291 (2023).
Luo, Y. et al. Myelocytomatosis-protein arginine n-methyltransferase 5 axis defines the tumourigenesis and immune response in hepatocellular carcinoma. Hepatology 74, 1932–1951 (2021).
Shivapurkar, N. et al. Treatment with a cholecystokinin receptor antagonist, proglumide, improves efficacy of immune checkpoint antibodies in hepatocellular carcinoma. Int. J. Mol. Sci. 24, 3625 (2023).
Xiong, Z. et al. Targeting PPAR-gamma counteracts tumour adaptation to immune-checkpoint blockade in hepatocellular carcinoma. Gut 72, 1758–1773 (2023).
Wang, L. et al. Targeting N6-methyladenosine reader YTHDF1 with siRNA boosts antitumour immunity in NASH-HCC by inhibiting EZH2-IL-6 axis. J. Hepatol. 79, 1185–1200 (2023).
Yu, M. et al. PARG inhibition limits HCC progression and potentiates the efficacy of immune checkpoint therapy. J. Hepatol. 77, 140–151 (2022).
Lin M. et al. Targeting fibrinogen-like protein 1 enhances immunotherapy in hepatocellular carcinoma. J. Clin. Invest. 133e164528 (2023).
Sukowati, C. et al. PD-L1 downregulation and DNA methylation inhibition for molecular therapy against cancer stem cells in hepatocellular carcinoma. Int J. Mol. Sci. 24, 13357 (2023).
Weng, J. et al. Intratumoural PPT1-positive macrophages determine immunosuppressive contexture and immunotherapy response in hepatocellular carcinoma. J. Immunother. Cancer 11, e006655 (2023).
Yang, S. F. et al. Neoantigen vaccination augments antitumour effects of anti-PD-1 on mouse hepatocellular carcinoma. Cancer Lett. 563, 216192 (2023).
Zhang, L. et al. DBF4 dependent kinase inhibition suppresses hepatocellular carcinoma progression and potentiates anti-programmed cell death-1 therapy. Int J. Biol. Sci. 19, 3412–3427 (2023).
Zhu, G. Q. et al. CD36(+) cancer-associated fibroblasts provide immunosuppressive microenvironment for hepatocellular carcinoma via secretion of macrophage migration inhibitory factor. Cell Discov. 9, 25 (2023).
Salman, S. et al. HIF inhibitor 32-134D eradicates murine hepatocellular carcinoma in combination with anti-PD1 therapy. J. Clin. Invest. 132, e156774 (2022).
Sung P. S., et al. Intrahepatic inflammatory IgA(+)PD-L1(high) monocytes in hepatocellular carcinoma development and immunotherapy. J. Immunother. Cancer 10, e003618 (2022).
Wei, Y. et al. A FAK inhibitor boosts anti-PD1 immunotherapy in a hepatocellular carcinoma mouse model. Front. Pharm. 12, 820446 (2021).
Yu, Z. et al. Nano delivery of simvastatin targets liver sinusoidal endothelial cells to remodel tumour microenvironment for hepatocellular carcinoma. J. Nanobiotechnol. 20, 9 (2022).
Torrens, L. et al. Immunomodulatory effects of lenvatinib plus anti-programmed cell death protein 1 in mice and rationale for patient enrichment in hepatocellular carcinoma. Hepatology 74, 2652–2669 (2021).
Yang, W. et al. A selective HDAC8 inhibitor potentiates antitumour immunity and efficacy of immune checkpoint blockade in hepatocellular carcinoma. Sci. Transl. Med. 13, eaaz6804 (2021).
Yi, C. et al. Lenvatinib targets FGF receptor 4 to enhance antitumour immune response of anti-programmed cell death-1 in HCC. Hepatology 74, 2544–2560 (2021).
Liu, M. et al. Targeting monocyte-intrinsic enhancer reprogramming improves immunotherapy efficacy in hepatocellular carcinoma. Gut 69, 365–379 (2020).
Shigeta, K. et al. Dual programmed death receptor-1 and vascular endothelial growth factor receptor-2 blockade promotes vascular normalization and enhances antitumour immune responses in hepatocellular carcinoma. Hepatology 71, 1247–1261 (2020).
Zhu, H. et al. Oxaliplatin induces immunogenic cell death in hepatocellular carcinoma cells and synergizes with immune checkpoint blockade therapy. Cell Oncol. 43, 1203–1214 (2020).
Acknowledgements
The authors acknowledge the following financial support: European Research Council Grant ERC-AdG-2020-FIBCAN #101021417 (T.F.B. and Y.H.); ERC-2022-PoC1 CANDY #101069276 (T.F.B.), EU HORIZON-HLTH-2021-DISEASE-04-07 D-SOLVE #101057917 (T.F.B.), ARC Grant TheraHCC2.0 IHU201301187 (T.F.B.); the French National Research Agency RHU DELIVER (ANR-21-RHUS-0001) (T.F.B. and P.N.) and LABEX ANR-10-LABX-0028_HEPSYS (T.F.B); the US National Institute of Health (R01CA233794, Y.H., T.F.B), the University of Strasbourg Foundation and Alsace Cancer Foundation (T.F.B). This work of the Interdisciplinary Thematic Institute IMCBio, as part of the ITI 2021-2028 program of the University of Strasbourg, CNRS and Inserm, was further supported by IdEx Unistra (ANR-10-IDEX-0002), and by SFRI-STRAT’US project (ANR 20-SFRI-0012) and EUR IMCBio (ANR-17-EURE-0023) under the framework of the French Investments for the Future Program and the France 2030 program. The figures have been created using BioRender.com.
Author information
Authors and Affiliations
Contributions
R.D. wrote the first draft of the manuscript and assembled the figures. F.G. assembled Table 1. A.S., Y.H., M.H., and P.N. revised and edited the manuscript. T.F.B. led the project and participated in the writing and revisions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Disclosures
Inserm, the University of Strasbourg, the Strasbourg University Hospitals and the IHU Strasbourg have filed patent applications for the use of anti-claudin-1 monoclonal antibodies for the treatment of fibrosis and cancers (T.F.B. inventor) which have been licensed to Alentis Therapeutics. The same organisations have also filed patent applications for a method for diagnosis and/or prognosis of liver disease progression and risk of hepatocellular carcinoma and discovery of therapeutic compounds and targets to treat liver disease and cancer as well as a clinical gene signature-based human cell culture model and uses thereof (T.F.B and Y.H. co-inventors). T.F.B. is founder, shareholder, and advisor for Alentis Therapeutics. P.N. has received honoraria from and/or consults for AstraZeneca, Bayer, Bristol-Myers Squibb, Eisai, Gilead, Guerbet, Ipsen, and Roche. He has also received research grants from AstraZeneca, AbbVie, Bristol-Myers Squibb, and Eisai.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Desert, R., Gianonne, F., Saviano, A. et al. Improving immunotherapy for the treatment of hepatocellular carcinoma: learning from patients and preclinical models. npj Gut Liver 2, 8 (2025). https://doi.org/10.1038/s44355-025-00018-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44355-025-00018-y