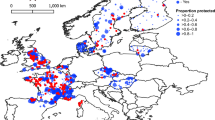
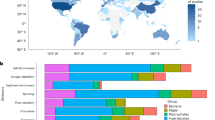
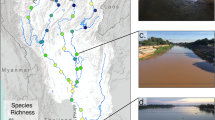
Abstract
To address the losses of river biodiversity worldwide, various conservation actions have been implemented to promote recovery of species and ecosystems. In this Review, we assess the effectiveness of these actions globally and regionally, and identify causes of success and failure. Overall, actions elicit little improvement in river biodiversity, in contrast with reports from terrestrial and marine ecosystems. This lack of improvement does not necessarily indicate a failure of any individual action. Rather, it can be attributed in part to remaining unaddressed stressors driving biodiversity loss; a poor match between the spatial scale of action and the scale of the affected area; and absence of adequate monitoring, including insufficient timescales, missing reference and control sites or insufficient selection of targeted taxa. Furthermore, outcomes are often not reported and are unevenly distributed among actions, regions and organism groups. Expanding from local-scale actions to coordinated, transformative, catchment-scale management approaches shows promise for improving outcomes. Such approaches involve identifying major stressors, appropriate conservation actions and source populations for recolonization, as well as comprehensive monitoring, relevant legislation and engaging all stakeholders to promote the recovery of river biodiversity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Everard, M. What have rivers ever done for us? Ecosystem services and river systems. In River Conservation and Management (eds Boon, P. J. & Raven, P. J) Ch. 25 (John Wiley & Sons, 2012).
Feio, M. J. et al. Fish and macroinvertebrate assemblages reveal extensive degradation of the world’s rivers. Glob. Change Biol. 29, 355–374 (2023).
Reid, A. J. et al. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 94, 849–873 (2019).
Langhammer, P. F. et al. The positive impact of conservation action. Science 384, 453–458 (2024).
Tickner, D. et al. Bending the curve of global freshwater biodiversity loss: an emergency recovery plan. BioScience 70, 330–342 (2020).
Lake, P. S., Bond, N. & Reich, P. Linking ecological theory with stream restoration. Freshw. Biol. 52, 597–615 (2007).
Britton, J. R. et al. Preventing and controlling nonnative species invasions to bend the curve of global freshwater biodiversity loss. Environ. Rev. 31, 310–326 (2023).
Sutherland, W. J., Pullin, A. S., Dolman, P. M. & Knight, T. M. The need for evidence-based conservation. Trends Ecol. Evol. 19, 305–308 (2004).
Bernhardt, E. S. & Palmer, M. A. River restoration: the fuzzy logic of repairing reaches to reverse catchment scale degradation. Ecol. Appl. 21, 1926–1931 (2011).
Ockendon, N. et al. Effectively integrating experiments into conservation practice. Ecol. Solut. Evid. 2, e12069 (2021).
Brudvig, L. A. & Catano, C. P. Prediction and uncertainty in restoration science. Restor. Ecol. 32, e13380 (2021).
Dawson, N. M. et al. Reviewing the science on 50 years of conservation: knowledge production biases and lessons for practice. Ambio 53, 1395–1413 (2024).
Toszogyova, A., Smyčka, J. & Storch, D. Mathematical biases in the calculation of the Living Planet Index lead to overestimation of vertebrate population decline. Nat. Commun. 15, 5295 (2024).
Torres-Romero, E. J., Fisher, J. T., Nijman, V., He, F. & Eppley, T. M. Accelerated human-induced extinction crisis in the world’s freshwater mammals. Glob. Environ. Change Adv. 2, 100006 (2024).
He, F. et al. The global decline of freshwater megafauna. Glob. Change Biol. 25, 3883–3892 (2019).
Deinet, S. et al. The Living Planet Index (LPI) for migratory freshwater fish. World Fish Migration Foundation http://worldfishmigrationfoundation.com/wp-content/uploads/2020/07/LPI_report_2020.pdf (2020).
Jaureguiberry, P. et al. The direct drivers of recent global anthropogenic biodiversity loss. Sci. Adv. 8, eabm9982 (2022).
Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. IPBES https://www.ipbes.net/global-assessment (2019).
Gallardo, B., Clavero, M., Sánchez, M. I. & Vilà, M. Global ecological impacts of invasive species in aquatic ecosystems. Glob. Change Biol. 22, 151–163 (2016).
Jakubínský, J. et al. Managing floodplains using nature‐based solutions to support multiple ecosystem functions and services. WIREs Water 8, e1545 (2021).
Van Rees, C. B. et al. The potential for nature-based solutions to combat the freshwater biodiversity crisis. PLOS Water 2, e0000126 (2023).
Cohen-Shacham, E., Walters, G., Janzen, C. & Maginnis, S. Nature-based solutions to address global societal challenges. IUCN https://portals.iucn.org/library/sites/library/files/documents/2016-036.pdf (2016).
Van Rees, C. B. et al. An interdisciplinary overview of levee setback benefits: supporting spatial planning and implementation of riverine nature‐based solutions. WIREs Water 11, e1750 (2024).
Palmer, M. A., Menninger, H. L. & Bernhardt, E. River restoration, habitat heterogeneity and biodiversity: a failure of theory or practice? Freshw. Biol. 55, 205–222 (2010).
Bellmore, J. R. et al. Conceptualizing ecological responses to dam removal: if you remove it, what’s to come? BioScience 69, 26–39 (2019).
Silva, A. T. et al. The future of fish passage science, engineering, and practice. Fish. Fish 19, 340–362 (2018).
Roni, P., Hanson, K. & Beechie, T. Global review of the physical and biological effectiveness of stream habitat rehabilitation techniques. North. Am. J. Fish. Manag. 28, 856–890 (2008).
Al-Zankana, A. F. A., Matheson, T. & Harper, D. M. How strong is the evidence—based on macroinvertebrate community responses—that river restoration works? Ecohydrol. Hydrobiol. 20, 196–214 (2020).
Kail, J., Brabec, K., Poppe, M. & Januschke, K. The effect of river restoration on fish, macroinvertebrates and aquatic macrophytes: a meta-analysis. Ecol. Indic. 58, 311–321 (2015).
Wohl, E., Lane, S. N. & Wilcox, A. C. The science and practice of river restoration. Water Resour. Res. 51, 5974–5997 (2015).
Lu, W., Arias Font, R., Cheng, S., Wang, J. & Kollmann, J. Assessing the context and ecological effects of river restoration—a meta-analysis. Ecol. Eng. 136, 30–37 (2019).
Sundermann, A., Stoll, S. & Haase, P. River restoration success depends on the species pool of the immediate surroundings. Ecol. Appl. 21, 1962–1971 (2011).
Rogosch, J. S. et al. Evaluating effectiveness of restoration to address current stressors to riverine fish. Freshw. Biol. 69, 607–622 (2024).
Atristain, M., Solagaistua, L., Larrañaga, A., Von Schiller, D. & Elosegi, A. Slow drawdown, fast recovery: stream macroinvertebrate communities improve quickly after large dam decommissioning. J. Appl. Ecol. 61, 1481–1491 (2024).
Hansen, J. F. & Hayes, D. B. Long-term implications of dam removal for macroinvertebrate communities in Michigan and Wisconsin rivers, United States. River Res. Appl. 28, 1540–1550 (2012).
Poulos, H. M. et al. Dam removal effects on benthic macroinvertebrate dynamics: a New England stream case study (Connecticut, USA). Sustainability 11, 2875 (2019).
Roscoe, D. W. & Hinch, S. G. Effectiveness monitoring of fish passage facilities: historical trends, geographic patterns and future directions. Fish. Fish 11, 12–33 (2010).
Pompeu, P. S., Agostinho, A. A. & Pelicice, F. M. Existing and future challenges: the concept of successful fish passage in South America. River Res. Appl. 28, 504–512 (2012).
Lira, N. A. et al. Fish passages in South America: an overview of studied facilities and research effort. Neotropical Ichthyol. 15, https://doi.org/10.1590/1982-0224-20160139 (2017).
Arthington, A. H. et al. Accelerating environmental flow implementation to bend the curve of global freshwater biodiversity loss. Environ. Rev. 32, 387–413 (2023).
Messager, M. L. et al. A metasystem approach to designing environmental flows. BioScience 73, 643–662 (2023).
Datry, T. et al. Causes, responses, and implications of anthropogenic versus natural flow intermittence in river networks. BioScience 73, 9–22 (2023).
Acreman, M. et al. Environmental flows for natural, hybrid, and novel riverine ecosystems in a changing world. Front. Ecol. Environ. 12, 466–473 (2014).
Dourado, G. F., Rallings, A. M. & Viers, J. H. Overcoming persistent challenges in putting environmental flow policy into practice: a systematic review and bibliometric analysis. Environ. Res. Lett. 18, 043002 (2023).
Cheng, L. et al. Managing the three gorges dam to implement environmental flows in the Yangtze River. Front. Environ. Sci. 6, 64 (2018).
Pander, J., Knott, J., Mueller, M. & Geist, J. Effects of environmental flows in a restored floodplain system on the community composition of fish, macroinvertebrates and macrophytes. Ecol. Eng. 132, 75–86 (2019).
Kiernan, J. D., Moyle, P. B. & Crain, P. K. Restoring native fish assemblages to a regulated California stream using the natural flow regime concept. Ecol. Appl. 22, 1472–1482 (2012).
Salinas-Rodríguez, S. et al. What do environmental flows mean for long-term freshwater ecosystems’ protection? Assessment of the mexican water reserves for the environment program. Sustainability 13, 1240 (2021).
Schlatter, K. J., Grabau, M. R., Shafroth, P. B. & Zamora-Arroyo, F. Integrating active restoration with environmental flows to improve native riparian tree establishment in the Colorado River Delta. Ecol. Eng. 106, 661–674 (2017).
Walter, C. A., Nelson, D. & Earle, J. I. Assessment of stream restoration: sources of variation in macroinvertebrate recovery throughout an 11‐year study of coal mine drainage treatment. Restor. Ecol. 20, 431–440 (2012).
Erős, T., Takács, P., Czeglédi, I., Sály, P. & Specziár, A. Taxonomic- and trait-based recolonization dynamics of a riverine fish assemblage following a large-scale human-mediated disturbance: the red mud disaster in Hungary. Hydrobiologia 758, 31–45 (2015).
Fritz, K. M. et al. Structural and functional characteristics of natural and constructed channels draining a reclaimed mountaintop removal and valley fill coal mine. J. North. Am. Benthol. Soc. 29, 673–689 (2010).
Pond, G. J. et al. Long-term impacts on macroinvertebrates downstream of reclaimed mountaintop mining valley fills in central Appalachia. Environ. Manag. 54, 919–933 (2014).
Palmer, M. A. & Hondula, K. L. Restoration as mitigation: analysis of stream mitigation for coal mining impacts in southern Appalachia. Environ. Sci. Technol. 48, 10552–10560 (2014).
Jones, E. R., Van Vliet, M. T. H., Qadir, M. & Bierkens, M. F. P. Country-level and gridded estimates of wastewater production, collection, treatment and reuse. Earth Syst. Sci. Data 13, 237–254 (2021).
Boets, P. et al. Do investments in water quality and habitat restoration programs pay off? An analysis of the chemical and biological water quality of a lowland stream in the Zwalm River basin (Belgium). Environ. Sci. Policy 124, 115–124 (2021).
Kesler, M., Kangur, M. & Vetemaa, M. Natural re‐establishment of Atlantic salmon reproduction and the fish community in the previously heavily polluted River Purtse, Baltic Sea. Ecol. Freshw. Fish. 20, 472–477 (2011).
Gibson-Reinemer, D. K. et al. Ecological recovery of a river fish assemblage following the implementation of the Clean Water Act. BioScience 67, 957–970 (2017).
Artz, C., Pyron, M. & Bowley, L. Long-term macroinvertebrate assemblages of the West Fork White River, Indiana improve following the Clean Water Act. Am. Midl. Nat. 184, 233–247 (2020).
Dyer, S. D. & Wang, X. A comparison of stream biological responses to discharge from wastewater treatment plants in high and low population density areas. Environ. Toxicol. Chem. 21, 1065–1075 (2002).
Jones, E. R. et al. Current wastewater treatment targets are insufficient to protect surface water quality. Commun. Earth Environ. 3, 221 (2022).
Trejos Delgado, C., Dombrowski, A. & Oehlmann, J. Assessing the impact of two conventional wastewater treatment plants on small streams with effect-based methods. PeerJ 12, e17326 (2024).
Muñoz, I. et al. Effects of emerging contaminants on biodiversity, community structure, and adaptation of River Biota. In Emerging Contaminants in River Ecosystems (eds Petrovic, M. et al.) 79–119 (Springer, 2015).
Khasawneh, O. F. S. & Palaniandy, P. Occurrence and removal of pharmaceuticals in wastewater treatment plants. Process. Saf. Environ. Prot. 150, 532–556 (2021).
Rout, P. R., Zhang, T. C., Bhunia, P. & Surampalli, R. Y. Treatment technologies for emerging contaminants in wastewater treatment plants: a review. Sci. Total. Environ. 753, 141990 (2021).
Owolabi, T. A., Mohandes, S. R. & Zayed, T. Investigating the impact of sewer overflow on the environment: a comprehensive literature review paper. J. Environ. Manag. 301, 113810 (2022).
Kayhanian, M. Trend and concentrations of legacy lead (Pb) in highway runoff. Environ. Pollut. 160, 169–177 (2012).
Palt, M., Hering, D. & Kail, J. Context‐specific positive effects of woody riparian vegetation on aquatic invertebrates in rural and urban landscapes. J. Appl. Ecol. 60, 1010–1021 (2023).
Klein, M. et al. Risk mitigation measures for pesticide runoff: how effective are they? Pest. Manag. Sci. 79, 4897–4905 (2023).
Stoddard, J. L. et al. Regional trends in aquatic recovery from acidification in North America and Europe. Nature 401, 575–578 (1999).
Sullivan, T. J. et al. Air pollution success stories in the United States: the value of long-term observations. Environ. Sci. Policy 84, 69–73 (2018).
Lin, J. et al. Clean Air Act policies reduced stream nitrogen concentrations over time in deposition dominated watersheds of the conterminous US (2000–2014). EPA https://assessments.epa.gov/risk/document/&deid%3D350435 (2020).
Murphy, J. F. et al. Evidence of recovery from acidification in the macroinvertebrate assemblages of UK fresh waters: a 20-year time series. Ecol. Indic. 37, 330–340 (2014).
Pharaoh, E., Diamond, M., Ormerod, S. J., Rutt, G. & Vaughan, I. P. Evidence of biological recovery from gross pollution in English and Welsh rivers over three decades. Sci. Total. Environ. 878, 163107 (2023).
Vaughan, I. P. & Ormerod, S. J. Large‐scale, long‐term trends in British river macroinvertebrates. Glob. Change Biol. 18, 2184–2194 (2012).
Baker, N. J., Pilotto, F., Jourdan, J., Beudert, B. & Haase, P. Recovery from air pollution and subsequent acidification masks the effects of climate change on a freshwater macroinvertebrate community. Sci. Total. Environ. 758, 143685 (2021).
Qu, Y. et al. Significant improvement in freshwater invertebrate biodiversity in all types of English rivers over the past 30 years. Sci. Total. Environ. 905, 167144 (2023).
Fraker, M. E. et al. Projecting the effects of agricultural conservation practices on stream fish communities in a changing climate. Sci. Total. Environ. 747, 141112 (2020).
Tibbetts, J., Krause, S., Lynch, I. & Sambrook Smith, G. H. Abundance, distribution, and drivers of microplastic contamination in urban river environments. Water 10, 1597 (2018).
Kukkola, A. et al. Prevailing impacts of river management on microplastic transport in contrasting US streams: rethinking global microplastic flux estimations. Water Res. 240, 120112 (2023).
Lorion, C. M. & Kennedy, B. P. Relationships between deforestation, riparian forest buffers and benthic macroinvertebrates in neotropical headwater streams. Freshw. Biol. 54, 165–180 (2009).
Collins, K. E., Doscher, C., Rennie, H. G. & Ross, J. G. The effectiveness of riparian ‘restoration’ on water quality—a case study of lowland streams in Canterbury, New Zealand. Restor. Ecol. 21, 40–48 (2013).
Koehnken, L. et al. Impacts of riverine sand mining on freshwater ecosystems: a review of the scientific evidence and guidance for future research. River Res. Appl. 36, 362–370 (2020).
Dankel, D. J., Skagen, D. W. & Ulltang, Ø. Fisheries management in practice: review of 13 commercially important fish stocks. Rev. Fish Biol. Fish. 18, 201–233 (2008).
Arostegui, M. C. et al. Approaches to regulating recreational fisheries: balancing biology with angler satisfaction. Rev. Fish Biol. Fish. 31, 573–598 (2021).
Cowx, I. G. Characterisation of inland fisheries in Europe. Fish. Manag. Ecol. 22, 78–87 (2015).
Hunt, T. L. & Jones, P. Informing the great fish stocking debate: an Australian case study. Rev. Fish. Sci. Aquac. 26, 275–308 (2018).
White, J. et al. Incorporating conservation limit variability and stock risk assessment in precautionary salmon catch advice at the river scale. ICES J. Mar. Sci. 80, 803–822 (2023).
Acuña-Alonso, C., Varandas, S., Álvarez, X. & Martinho, A. Analysis of the evolution of a fisheries management plan based on environmental governance: living laboratory in the Olo River, Portugal. Fish. Res. 260, 106595 (2023).
Castello, L., Viana, J. P., Watkins, G., Pinedo-Vasquez, M. & Luzadis, V. A. Lessons from integrating fishers of arapaima in small-scale fisheries management at the Mamirauá Reserve, Amazon. Environ. Manag. 43, 197–209 (2009).
Cote, D., Van Leeuwen, T. E., Bath, A. J., Gonzales, E. K. & Cote, A. L. Social–ecological management results in sustained recovery of an imperiled salmon population. Restor. Ecol. 29, e13401 (2021).
Dadswell, M. et al. The decline and impending collapse of the Atlantic salmon (Salmo salar) population in the North Atlantic Ocean: a review of possible causes. Rev. Fish. Sci. Aquac. 30, 215–258 (2022).
Andrew King, R., Miller, A. L. & Stevens, J. R. Has stocking contributed to an increase in the rod catch of anadromous trout (Salmo trutta L.) in the Shetland Islands, UK? J. Fish. Biol. 99, 980–989 (2021).
Righi, T., Fasola, E., Iaia, M., Stefani, F. & Volta, P. Limited contribution of hatchery-produced individuals to the sustainment of wild marble trout (Salmo marmoratus Cuvier, 1829) in an Alpine basin. Sci. Total. Environ. 892, 164555 (2023).
Baer, J., Blasel, K. & Diekmann, M. Benefits of repeated stocking with adult, hatchery‐reared brown trout, Salmo trutta, to recreational fisheries? Fish. Manag. Ecol. 14, 51–59 (2007).
Hulme, P. E. Beyond control: wider implications for the management of biological invasions. J. Appl. Ecol. 43, 835–847 (2006).
Olden, J. D., Whattam, E. & Wood, S. A. Online auction marketplaces as a global pathway for aquatic invasive species. Hydrobiologia 848, 1967–1979 (2021).
Yick, J. L., Wisniewski, C., Diggle, J. & Patil, J. G. Eradication of the invasive common carp, Cyprinus carpio from a large lake: lessons and insights from the Tasmanian experience. Fishes 6, 6 (2021).
Fausch, K. D., Rieman, B. E., Dunham, J. B., Young, M. K. & Peterson, D. P. Invasion versus isolation: trade‐offs in managing native salmonids with barriers to upstream movement. Conserv. Biol. 23, 859–870 (2009).
Jones, P. E. et al. The use of barriers to limit the spread of aquatic invasive animal species: a global review. Front. Ecol. Evol. 9, 611631 (2021).
Leung, B. et al. An ounce of prevention or a pound of cure: bioeconomic risk analysis of invasive species. Proc. R. Soc. Lond. B 269, 2407–2413 (2002).
Hussner, A. et al. Management and control methods of invasive alien freshwater aquatic plants: a review. Aquat. Bot. 136, 112–137 (2017).
Gosling, L. M. & Baker, S. J. The eradication of muskrats and coypus from Britain. Biol. J. Linn. Soc. 38, 39–51 (1989).
Bryce, R. et al. Turning back the tide of American mink invasion at an unprecedented scale through community participation and adaptive management. Biol. Conserv. 144, 575–583 (2011).
Caffrey, J. Rapid response achieves eradication—chub in Ireland. Manag. Biol. Invasions 9, 475–482 (2018).
Van Der Walt, J. A. et al. Successful mechanical eradication of spotted bass (Micropterus punctulatus (Rafinesque, 1819)) from a South African river. Aquat. Conserv. Mar. Freshw. Ecosyst. 29, 303–311 (2019).
Lintermans, M. Recolonization by the mountain galaxias Galaxias olidus of a montane stream after the eradication of rainbow trout Oncorhynchus mykiss. Mar. Freshw. Res. 52, 257 (2001).
Weyl, O. L. F., Finlayson, B., Impson, N. D., Woodford, D. J. & Steinkjer, J. Threatened endemic fishes in South Africa’s Cape floristic region: a new beginning for the Rondegat River. Fisheries 39, 270–279 (2014).
June-Wells, M. et al. Seventeen years of grass carp: an examination of vegetation management and collateral impacts in Ball Pond, New Fairfield, Connecticut. Lake Reserv. Manag. 33, 84–100 (2017).
Dalu, T. et al. Ecosystem responses to the eradication of common carp Cyprinus carpio using rotenone from a reservoir in South Africa. Aquat. Conserv. Mar. Freshw. Ecosyst. 30, 2284–2297 (2020).
Dibble, E. D. & Kovalenko, K. Ecological impact of grass carp: a review of the available data. J Aquat. Plant Manag. 47, 1–15 (2009).
Gherardi, F., Aquiloni, L., Diéguez-Uribeondo, J. & Tricarico, E. Managing invasive crayfish: is there a hope? Aquat. Sci. 73, 185–200 (2011).
Rytwinski, T. et al. The effectiveness of non-native fish removal techniques in freshwater ecosystems: a systematic review. Environ. Rev. 27, 71–94 (2019).
Moorhouse, T. P. & Macdonald, D. W. Are invasives worse in freshwater than terrestrial ecosystems? WIREs Water 2, 1–8 (2015).
Kurylyk, B. L., MacQuarrie, K. T. B., Linnansaari, T., Cunjak, R. A. & Curry, R. A. Preserving, augmenting, and creating cold‐water thermal refugia in rivers: concepts derived from research on the Miramichi River, New Brunswick (Canada). Ecohydrology 8, 1095–1108 (2015).
Feng, M., Zolezzi, G. & Pusch, M. Effects of thermopeaking on the thermal response of alpine river systems to heatwaves. Sci. Total. Environ. 612, 1266–1275 (2018).
Salerno, F., Gaetano, V. & Gianni, T. Urbanization and climate change impacts on surface water quality: enhancing the resilience by reducing impervious surfaces. Water Res. 144, 491–502 (2018).
Moore, T. L., Gulliver, J. S., Stack, L. & Simpson, M. H. Stormwater management and climate change: vulnerability and capacity for adaptation in urban and suburban contexts. Clim. Change 138, 491–504 (2016).
Rahel, F. J. Managing freshwater fish in a changing climate: resist, accept, or direct. Fisheries 47, 245–255 (2022).
Bond, N. R., Thomson, J. R. & Reich, P. Incorporating climate change in conservation planning for freshwater fishes. Divers. Distrib. 20, 931–942 (2014).
Palmer, M. A. et al. Climate change and the world’s river basins: anticipating management options. Front. Ecol. Environ. 6, 81–89 (2008).
Wilby, R. L. et al. Evidence needed to manage freshwater ecosystems in a changing climate: turning adaptation principles into practice. Sci. Total. Environ. 408, 4150–4164 (2010).
Williams, J. E., Isaak, D. J., Imhof, J., Hendrickson, D. A. & McMillan, J. R. Cold-water fishes and climate change in North America. In Reference Module in Earth Systems and Environmental Sciences 1–10 (Elsevier, 2015).
Abell, R., Allan, J. D. & Lehner, B. Unlocking the potential of protected areas for freshwaters. Biol. Conserv. 134, 48–63 (2007).
Gonçalves, D. V. & Hermoso, V. Global goals overlook freshwater conservation. Science 377, 380–380 (2022).
Caldwell, I. R. et al. Global trends and biases in biodiversity conservation research. Cell Rep. Sustain. 1, 100082 (2024).
Leal, C. G. et al. Integrated terrestrial-freshwater planning doubles conservation of tropical aquatic species. Science 370, 117–121 (2020).
Mancini, L. et al. Biological quality of running waters in protected areas: the influence of size and land use. Biodivers. Conserv. 14, 351–364 (2005).
Nogueira, J. G., Teixeira, A., Varandas, S., Lopes‐Lima, M. & Sousa, R. Assessment of a terrestrial protected area for the conservation of freshwater biodiversity. Aquat. Conserv. Mar. Freshw. Ecosyst. 31, 520–530 (2021).
Acreman, M., Hughes, K. A., Arthington, A. H., Tickner, D. & Dueñas, M. Protected areas and freshwater biodiversity: a novel systematic review distils eight lessons for effective conservation. Conserv. Lett. 13, e12684 (2020).
Hermoso, V., Filipe, A. F., Segurado, P. & Beja, P. Filling gaps in a large reserve network to address freshwater conservation needs. J. Environ. Manag. 161, 358–365 (2015).
Azevedo‐Santos, V. M. et al. Protected areas: a focus on Brazilian freshwater biodiversity. Divers. Distrib. 25, 442–448 (2019).
Cid, N. et al. From meta‐system theory to the sustainable management of rivers in the Anthropocene. Front. Ecol. Environ. 20, 49–57 (2022).
Zhang, H., Wang, Q., Li, G., Zhang, H. & Zhang, J. Losses of ecosystem service values in the Taihu Lake Basin from 1979 to 2010. Front. Earth Sci. 11, 310–320 (2017).
Dudley, N., Parrish, J. D., Redford, K. H. & Stolton, S. The revised IUCN protected area management categories: the debate and ways forward. Oryx 44, 485–490 (2010).
Mascia, M. B. et al. Protected area downgrading, downsizing, and degazettement (PADDD) in Africa, Asia, and Latin America and the Caribbean, 1900–2010. Biol. Conserv. 169, 355–361 (2014).
European Red List of Habitats. Part 2: Terrestrial and Freshwater Habitats. European Commission: Directorate-General for Environment https://op.europa.eu/en/publication-detail/-/publication/22542b64-c501-11e7-9b01-01aa75ed71a1/language-en (2016).
Nel, J. L., Reyers, B., Roux, D. J. & Cowling, R. M. Expanding protected areas beyond their terrestrial comfort zone: identifying spatial options for river conservation. Biol. Conserv. 142, 1605–1616 (2009).
Hermoso, V., Vasconcelos, R. P., Henriques, S., Filipe, A. F. & Carvalho, S. B. Conservation planning across realms: enhancing connectivity for multi‐realm species. J. Appl. Ecol. 58, 644–654 (2021).
Cooke, S. J. et al. Is it a new day for freshwater biodiversity? Reflections on outcomes of the Kunming–Montreal Global Biodiversity Framework. PLOS Sustain. Transform. 2, e0000065 (2023).
Kura, Y. et al. Conservation for sustaining livelihoods: adaptive co-management of fish no-take zones in the Mekong River. Fish. Res. 265, 106744 (2023).
Koning, A. A., Perales, K. M., Fluet-Chouinard, E. & McIntyre, P. B. A network of grassroots reserves protects tropical river fish diversity. Nature 588, 631–635 (2020).
Jumani, S., Hull, V., Dandekar, P. & Mahesh, N. Community-based fish sanctuaries: untapped potential for freshwater fish conservation. Oryx 57, 522–531 (2023).
Geldmann, J., Manica, A., Burgess, N. D., Coad, L. & Balmford, A. A global-level assessment of the effectiveness of protected areas at resisting anthropogenic pressures. Proc. Natl. Acad. Sci. 116, 23209–23215 (2019).
The IUCN Red List of Threatened Species. IUCN www.iucnredlist.org (2024).
The EU birds and habitats directives—for nature and people in Europe. European Commission: Directorate-General for Environment https://op.europa.eu/en/publication-detail/-/publication/7230759d-f136-44ae-9715-1eacc26a11af (2015).
Report to Congress on the Recovery of Threatened and Endangered Species Fiscal Years 2017–2020. United States Fish and Wildlife Service www.fws.gov/media/report-congress-recovery-threatened-and-endangered-species-fiscal-years-2017-2020 (2022).
Tsakiris, E. T., Randklev, C. R., Blair, A., Fisher, M. & Conway, K. W. Effects of translocation on survival and growth of freshwater mussels within a West Gulf Coastal Plain river system. Aquat. Conserv. Mar. Freshw. Ecosyst. 27, 1240–1250 (2017).
Vught, I., De Charleroy, D., Van Liefferinge, C., Coenen, E. & Coeck, J. Conservation of bullhead Cottus perifretum in the Demer River (Belgium) basin using re-introduction. J. Appl. Ichthyol. 27, 60–65 (2011).
Thiem, J. D. et al. Recovery from a fish kill in a semi‐arid Australian river: can stocking augment natural recruitment processes? Austral Ecol. 42, 218–226 (2017).
Betts, J. et al. A framework for evaluating the impact of the IUCN Red List of threatened species. Conserv. Biol. 34, 632–643 (2020).
Sayer, C. A. et al. One-quarter of freshwater fauna threatened with extinction. Nature https://doi.org/10.1038/s41586-024-08375-z (2025).
Lopes-Lima, M. et al. Major shortfalls impairing knowledge and conservation of freshwater molluscs. Hydrobiologia 848, 2831–2867 (2021).
Bourlat, S. J., Tschan, G. F., Martin, S., Iqram, M. & Leidenberger, S. A red listing gap analysis of molluscs and crustaceans in Northern Europe: what has happened in the last 10 years? Biol. Conserv. 286, 110247 (2023).
Chilcott, S. et al. Extinct habitat, extant species: lessons learned from conservation recovery actions for the Pedder galaxias (Galaxias pedderensis) in south-west Tasmania, Australia. Mar. Freshw. Res. 64, 864 (2013).
Moy, K. et al. Alternative conservation outcomes from aquatic fauna translocations: losing and saving the Running River rainbowfish. Aquat. Conserv. Mar. Freshw. Ecosyst. 33, 1445–1459 (2023).
Worthington, T. et al. The re-introduction of the burbot to the United Kingdom and Flanders. In Global Re-Introduction Perspectives: Re-Introduction Case-Studies from Around the Globe (ed. Soorae, P. S.) 284 (IUCN/SSC Re-introduction Specialist Group, 2008).
Kubota, H., Watanabe, K., Sakai, T. & Takahashi, T. Supportive breeding of the Tokyo bitterling in Tochigi Prefecture, Japan. In Global Re-Introduction Perspectives: Additional Case-Studies from Around the Globe (ed. Soorae, P. S.) 352 (IUCN/SSC Re-introduction Specialist Group, 2010).
Jourdan, J. et al. Reintroduction of freshwater macroinvertebrates: challenges and opportunities: reintroduction of freshwater macroinvertebrates. Biol. Rev. 94, 368–387 (2019).
Watson, A. S. & Castillo, L. Are protected areas working for endangered frogs in the Peruvian Andes? Biodivers. Conserv. 31, 1847–1866 (2022).
Van Rijssel, J. C. et al. Reintroducing Atlantic salmon in the river Rhine for decades: why did it not result in the return of a viable population? River Res. Appl. 40, 1164–1182 (2024).
Rao, R. J. Supplementation of Indian Gharial in protected areas of Madhya Pradesh, India. In Global Re-Introduction Perspectives: Additional Case-Studies from Around the Globe (ed. Soorae, P. S.) 284 (IUCN/SSC Re-introduction Specialist Group, 2008).
Whiterod, N. S., Asmus, M., Zukowski, S., Gilligan, D. & Daly, T. Reintroduction to re-establish locally extirpated populations of the Murray crayfish—second largest freshwater crayfish in the world—in S.E. Australia. In Global Conservation Translocation Perspectives: Case Studies From Around The Globe (ed. Soorae, P. S.) (IUCN/SSC Conservation Translocation Specialist Group, Environment Agency, 2021).
Haase, P. et al. The recovery of European freshwater biodiversity has come to a halt. Nature 620, 582–588 (2023).
Bunt, C. M., Castro‐Santos, T. & Haro, A. Performance of fish passage structures at upstream barriers to mitigation. River Res. Appl. 28, 457–478 (2012).
Vörösmarty, C. J. et al. Global threats to human water security and river biodiversity. Nature 467, 555–561 (2010).
Birk, S. et al. Impacts of multiple stressors on freshwater biota across spatial scales and ecosystems. Nat. Ecol. Evol. 4, 1060–1068 (2020).
Reich, P. et al. Aquatic invertebrate responses to riparian restoration and flow extremes in three degraded intermittent streams: an eight‐year field experiment. Freshw. Biol. 68, 325–339 (2023).
Sinclair, J. S., Mademann, J. A., Haubrock, P. J. & Haase, P. Primarily neutral effects of river restoration on macroinvertebrates, macrophytes, and fishes after a decade of monitoring. Restor. Ecol. 31, e13840 (2023).
Johnson, M. F. & Wilby, R. L. Seeing the landscape for the trees: metrics to guide riparian shade management in river catchments. Water Resour. Res. 51, 3754–3769 (2015).
Fausch, K. D., Torgersen, C. E., Baxter, C. V. & Li, H. W. Landscapes to riverscapes: bridging the gap between research and conservation of stream fishes. BioScience 52, 483 (2002).
Stoll, S., Breyer, P., Tonkin, J. D., Früh, D. & Haase, P. Scale-dependent effects of river habitat quality on benthic invertebrate communities—implications for stream restoration practice. Sci. Total. Environ. 553, 495–503 (2016).
Gonzalez, A. et al. Estimating local biodiversity change: a critique of papers claiming no net loss of local diversity. Ecology 97, 1949–1960 (2016).
White, E. R. Minimum time required to detect population trends: the need for long-term monitoring programs. BioScience 69, 40–46 (2019).
England, J. et al. Best practices for monitoring and assessing the ecological response to river restoration. Water 13, 3352 (2021).
Didham, R. K. et al. Interpreting insect declines: seven challenges and a way forward. Insect Conserv. Divers. 13, 103–114 (2020).
Bouwer, H. Integrated water management: emerging issues and challenges. Agric. Water Manag. 45, 217–228 (2000).
Palmer, M. A., Hondula, K. L. & Koch, B. J. Ecological restoration of streams and rivers: shifting strategies and shifting goals. Annu. Rev. Ecol. Evol. Syst. 45, 247–269 (2014).
Brachet, C. & Valensuela, D. A Handbook for Integrated Water Resources Management in Basins (Global Water Partnership/International Network of Basin Organizations, 2009).
Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for community action in the field of water policy. Water Framework Directive https://www.eea.europa.eu/policy-documents/directive-2000-60-ec-of (2000).
Hillman, M. Integrating knowledge: the key challenge for a new paradigm in river management. Geogr. Compass 3, 1988–2010 (2009).
Wyborn, C. et al. Co-producing sustainability: reordering the governance of science, policy, and practice. Annu. Rev. Environ. Resour. 44, 319–346 (2019).
Norström, A. V. et al. Principles for knowledge co-production in sustainability research. Nat. Sustain. 3, 182–190 (2020).
Regulation (EU) 2024/1991 of the European Parliament and of the Council of 24 June 2024 on nature restoration and amending Regulation (EU) 2022/869 (text with EEA relevance). EUR-Lex https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32024R1991 (2024).
Acknowledgements
Funding was provided by the EU Horizon 2020 project eLTER PLUS (grant agreement 871128 to P.H., D.C.-G. and J.S.S.) and by the Collaborative Research Centre RESIST funded by the DFG (German Research Foundation) (grant CRC 1439/2 – project number: 426547801 to P.H.). M.A.P. was supported by the US National Science Foundation (award DEB-1856200). F.H. acknowledges support from the Chinese Academy of Sciences (E355S122).
Author information
Authors and Affiliations
Contributions
Conceptualization: P.H., J.S.S. and R.B.S. Data curation: D.C.-G. & J.S.S. Funding acquisition: P.H. Investigation: P.H., D.C.-G., F.H., J.J., T.M., F.M.P., M.A.P., R.J.R., R.B.S., E.A.R.W. and J.S.S. Methodology: D.C.-G., J.S.S. and P.H. Supervision: P.H. Visualization: P.H., D.C.-G., J.J. and E.A.R.W. Writing (original draft preparation): P.H. and J.S.S. with contributions from D.C.-G., F.H., T.M., F.M.P., M.A.P., R.B.S. and E.A.R.W. Review and editing: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Biodiversity thanks Steve Ormerod and Charles van Rees for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Convention on International Trade in Endangered Species of Wild Fauna and Flora: https://cites.org/eng
Convention on Migratory Species: https://www.cms.int/
WASH: https://www.unwater.org/water-facts/wash-water-sanitation-and-hygiene
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Haase, P., Cortés-Guzmán, D., He, F. et al. Successes and failures of conservation actions to halt global river biodiversity loss. Nat. Rev. Biodivers. 1, 104–118 (2025). https://doi.org/10.1038/s44358-024-00012-x
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44358-024-00012-x
This article is cited by
-
Global habitat hotspots and extinction vulnerability of terrestrial vertebrates
Nature Sustainability (2026)