Abstract
Melanoma is an invasive type of skin cancer that may metastasize and cause death. Early identification and timely management of melanoma significantly increase the chance of successful treatment and reduce the mortality rate. Visual inspection of pigmented lesions is not objective, often necessitating additional diagnostic procedures such as dermoscopy, reflectance confocal microscopy and biopsy. Here, we developed a wireless, battery-free, chip-less patch that non-invasively measures bio-impedance of lesions and suspicious parts of skin to melanoma as a complementary, nonvisual, wearable device. The patch, in conjunction with personal electronics, can detect abnormal bioimpedances that are associated with the risk of melanoma and alert the need for medical consultation in the early stage of development. Our analytical and experimental results as well as a pilot human subject study showed that the wearable patch identified the difference in bioimpedance of pigmented lesions and healthy tissues on skin for early assessment of lesions.
Similar content being viewed by others
Introduction
Melanoma is the most malicious type of skin cancer, and its incidence has risen considerably over the last decades. In the United States alone, 97,610 new cases of melanoma were diagnosed in 2023, and mortality rate was 79901,2. Early identification and timely management increase the chance of successful treatment. Visual inspection of lesions on skin is the first step to detect risk of melanoma3. However, this evaluation is not objective and does not provide comprehensive information from the lesions, necessitating additional evaluation such as dermoscopy, reflectance confocal microscope and biopsy4. These evaluations are conducted by dermatologists and are not intended to be used by providers in primary care and patients at home. In addition, deep learning-based imaging methods are used in melanoma detection5,6,7. However, these techniques often rely on surface morphological features without deep physiological information about lesions. Optical spectroscopy along with artificial intelligence (AI) algorithms also detects melanoma, basal cell carcinoma, and squamous cell carcinoma6. For instance, MelaFind is an AI dermatology device that is based on multispectral image analysis. This device classifies melanocytic lesions through three dimensional morphological features and guide biopsy decisions; however, it was intended for clinical evaluation and may have low specifity8,9. MelaFind was used for patients aged 40 and above but low specificity, raising concerns about false positives and unnecessary biopsies9.
A need exists for an accessible, adjunct wearable device to be used by primary health-care providers and patients outside of specialized dermatology clinics to evaluate cutaneous lesions and alert the risk of melanoma in the early stage of development. Electrical properties of tissues provide valuable information about their health status10,11,12. Any change in skin tissue is associated with the change in electrophysiological parameters and electrical impedances, that are important for understanding the health condition of the skin13. Impedance-based sensing devices capture deeper physiological properties compared to visual assessment, offering a complementary, non-visual diagnostic method when visual assessment is challenging8. In addition, previous studies demonstrated that incorporating electrical impedance spectroscopy (EIS) into the diagnostic workflow improves sensitivity and clinical decision-making. The addition of EIS increased the correct biopsy decision rate from 59.9% to 71.0%, with a relative improvement of nearly 40% in diagnostically challenging cases14. When combined with short-term digital dermoscopy, EIS achieved 100% melanoma sensitivity while reducing the need for follow-up imaging by 46.9%, thereby enabling earlier intervention15. In large-scale evaluations, EIS improved biopsy accuracy for melanoma from 85.2% to 91.1% and severe dysplastic nevi from 69.3% to 79.1%, while also decreasing unnecessary biopsies of benign lesions16. Furthermore, EIS demonstrated higher diagnostic sensitivity (75%) compared to dermoscopy alone (66%)17. Additionally, we compared the limitations of optical methods (e.g., skin tone bias, class imbalance specificity) with the advantages of impedance sensing in Table 1, highlighting how impedance can complement existing techniques and addressing some of the challenges of optical-based models. For instance, evaluating electrical impedance of the skin through EIS was used to distinguish benign lesions and healthy tissues from melanoma tumors18,19. The presence of malignant tumors affects the cell shape, density, and perfusion and thus changes impedance of skin compared to healthy skin tissues in the vicinity of the tumor20. The sensitivity and positive predictive of EIS for cutaneous melanoma were reported to be 94% and 78%. SciBase® developed a benchtop EIS system called Nevisense for melanoma detection. Nevisense has a handheld probe and micro-invasive electrodes to be inserted into skin and measure the impedance of lesions21. The instrument is costly, minimally invasive and requires skilled professionals to operate, which makes them unsuitable for home use and primary care.
Wearable impedance-based platforms represent a promising noninvasive and affordable approach for assessing the electrical properties of biological tissues. These systems are being developed to enable portable, accessible solutions for tumor detection22,23. Despite their potential, achieving high quality imaging with wearable electrical impedance tomography (EIT) devices remains a significant challenge24. One major issue is the need to amplify the injected current to improve signal quality and spatial resolution, although increasing current beyond safe limits can pose risks such as muscular damage25. Furthermore, existing EIT systems26 often suffer from limited spatial resolution, making it difficult to detect small tumors with high accuracy.
Here, we developed a flexible, battery-free, chip-less patch that detects impedance changes through measurable shifts in resonance frequency, allowing differentiation between healthy and abnormal tissues. The patch is accessible, low cost and disposable, and can alert the need for professional diagnosis through measurement of bioimpedance of the lesions. Additionally, it may serve as a home monitoring device for high-risk patients, helping them track changes in suspicious lesions and prompting medical consultation. This technique allows quantitative analysis of lesion properties rather than relying on subjective visual analysis and provides numerical data and reduces operator dependency. By capturing these physiological changes, the device may reduce unnecessary biopsies that often arise from reliance on surface-level morphological assessments. The patch is placed over the suspicious parts of the skin and obtains the impedance data in conjunction with a reader module. We measured the impedance of pigmented lesions as well as healthy skin tissue at 2–3 cm from the center of the lesion. This will allow identification of abnormal impedances associated with melanoma. Our finite element analysis showed that the electrical current distribution in healthy skin tissue changes in the presence of malignant tumors. In addition, the shape of lesions on skin affects electrical properties of tissue; therefore, the impedance of a lesion depends on the size and stage of development27. Moreover, our human subject study showed that the patch can measure the impedance of pigmented lesions, which could range between 1.7 kΩ to 8 kΩ depending on the size of the lesions. We also compared the results on human subjects from the wireless patch to the data collected with a commercial EIS system as a reference.
Results
Principle of operation, fabrication, and analysis of wireless measurement of skin impedance
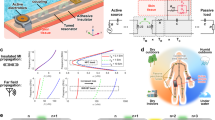
The illustration, photographs and the circuit diagram of a battery-free, flexible wireless patch and mechanism of sensing bioimpedance of tissues are presented in Fig. 1. The system consists of two separate parts: a bendable on-skin patch and a reader module highlighted in Fig. 1a, b and Supplementary Figs. 1, 2. The patch is placed on the skin for impedance measurement of healthy tissue and suspicious areas to melanoma. The electrodes of the patch are in contact with the tissue to measure the impedance as shown in Fig. 1a. A wireless link between coils on the reader module and patch enables the power delivery to the inductor-capacitor (LC) resonator within the patch, which is responsible for measuring the impedance of the human skin and lesion. The reader module has a power source and is placed 3 mm away from the patch and communicates wirelessly through magnetic induction. Electric current is induced through the patch by the reader. The magnetic coupling allows energy to be transferred from the reader to the patch, which then injects current into the skin through electrodes for impedance measurement. This mechanism allows the patch to operate without an on-board power source. A schematic view of the patch and photographs from the electrodes are presented in Fig. 1b. A 1-µm thick copper layer was screen printed onto a 100-µm thick polyimide substrate with the size 53 mm × 44 mm. A surface mounted capacitor with a size of 1 mm × 1 mm × 0.5 mm was soldered to the flexible patch as shown in Fig. 1b, c. Figure 1c shows a patch attached to skin with medical tape and the patch is bent. No rigid components exist on the interface of skin and patch, so the patch is comfortable on skin.
a Magnetic coupling between the patch on skin and the reader module and wireless injection of electrical current in healthy and cancerous tissue. b Detailed view of patch with resistor, capacitor, and inductor. The microscopic image of one electrode presented in the subset. c Photos of the patch showing it is bendable and is attached to the skin with medical tape. d Circuit diagram describing wireless measurement of human skin impedance. The circuit model for bioimpedance of skin tissue with intracellular current for high frequencies (CCM and RIC) and extracellular current at lower frequencies (REC).
A circuit diagram of the wearable patch is presented in Fig. 1d. The reader module has a primary resonator that magnetically couples with the secondary resonator on the patch to transmit power and read the impedance in a wireless mode. A parallel resistance-capacitance circuit RIC-CCM with REC represents an electrical model for skin tissues. The cell membrane of the human skin shows dielectric properties and can be modeled as electrical capacitance28. Low frequency currents pass through extracellular fluid and act as a resistor. In addition, human skin comprised of appendages (sweat gland and hair follicles), and lipid bilayer with embedded proteins characterized by hygroscopic property. Therefore, the skin appendages, extracellular fluids, and ion channel proteins to be resistors and skin cell membrane functions as capacitor11. The electrical impedance of the skin depends on several factors such as the presence of sweat gland, age of the individual, moisture content and environmental factors. In addition, damage to skin, wounds and existence of tumor and lesions affect the impedance. We used healthy skin tissue in the vicinity of lesions as a reference to mitigate the effect of unwanted parameters on impedance and reduce errors.
Electrical current is introduced into the reader module and creates an alternative magnetic flux in the primary coil, that magnetically couples into the coil on the patch and enables wireless power transmission. The resonant frequency (ωr) of the patch in connection to the skin is dependent on the patch capacitance (Cp), patch inductance (Lp) and skin capacitance (CCM)29,30:
The change in the capacitance of the skin will shift the resonant frequency of the patch that is detected on the reader side. The reflected impedance (Zr) is the impedance of the patch and is dependent on angular frequency (⍵), mutual induction (M) and combined impedance of the patch and the skin (ZpL) while the input impedance (Zin) is the overall impedance of the measurement system which is defined in Eqs. 2, 3.
Wireless measurement system is designed to resonate at an angular frequency (ω) of 5 MHz. Bioimpedance (ZL) of the skin causes the shift in the overall impedance of the patch and changes the reflected impedance (Zr). These changes impact the input impedance Zin (Eq.3) that is detected by the reader module where LR and CR is the inductance and capacitance of the reader module31.
Analysis of wireless impedance measurement, coupling coefficient, and sensitivity
The wireless measurement of impedance requires strong coupling for efficient power transfer and depends on the variation of bioimpedance (ZL) of skin. We analyzed a skin model to evaluate the sensitivity to coupling coefficient of the inductors as well as the resistance (REC) and capacitance (CCM) of skin tissue. The mutual inductance (M) between the primary inductor (LR) on the reader module and secondary inductor (LP) on the patch is defined as \(M=k\sqrt{{L}_{R}{L}_{P}}\), in which coupling coefficient (k) quantifies the extent of magnetic linkage between the two inductors31. A higher coupling coefficient indicates a stronger magnetic linkage and more efficient power transfer32. As the separation between the two coils is reduced, a larger voltage gain is observed in Fig. 2a and Supplementary Eq. 1. The maximum voltage gain at resonance frequency of 5.9 MHz increased from Gmax1 = 0.75 for k1 = 0.3 to Gmax2 = 1.4 for k2 = 0.5. This shows the distance between the reader module and the patch changes mutual inductance, and the voltage gain which affects the power transfer to the patch and sensitivity of the impedance measurement. Transfer function was calculated when primary (LR) and secondary inductors (LP) are 11.9 µH (LR = LP = 11.9 µH) and capacitors on the reader and patch are 82 pF (CR = CP = 82 pF). The internal impedance of the source was Zs = 50 Ω.
a Dependance of voltage gain of the circuit on coupling coefficient. b Power reflection S11 at different values of coupling coefficients. c Sensitivity of the patch on skin resistance at different coupling coefficients. d Sensitivity of the patch on skin capacitance at different coupling coefficients. e FEA of electrical current density on lesions for electrodes placed on the lesions and electrodes on the skin near the edge of the lesions. Distance between electrodes changed to apply currents to skin and lesions. f FEA of current density of the malignant and benign tumor. Color bar unit is in µA/mm2.
Figure 2b shows the effect of coupling coefficient k on power reflection (S11). Increasing k from 0.4 to 0.6 reduced S11 by 20 dB which means the amount of reflected power was reduced and more power was transferred to the patch. However, the value of S11 increased by 17 dB when coupling coefficient k increased from 0.6 to 0.8 resulting in more reflection and less power to the patch. This is because the coupling becomes sensitive to position and causes the splitting in the resonance frequency due to over coupling29. Optimal energy transfer between the skin and the patch is important for accurate measurement. Our findings show that coupling coefficient of 0.6 is the optimal value for efficient power transfer for this system. Additionally, power reflection (S11) is associated with the change in the input impedance (Zin) due to the change in bioimpedance of the skin33. S11 is calculated using Eq. 4 and Supplementary Eq. 6:
in which Zin represents the total input impedance of the measurement system, and Zs is the internal resistance of the source. The skin model has a resistance of 2 kΩ and capacitance of 8 pF. Sensitivity analysis was performed to study the output characteristics of impedance measurement system in response to the changes in skin model circuit capacitance (Fig. 2c and Supplementary Eq. 4) and resistance (Fig. 2d and Supplementary Eq. 5)34. The sensitivity to skin resistance (Eq. 5) and capacitance (Eq. 6) is defined as:
In Fig. 2c, when the skin model resistance (REC) increased from 2 to 10 kΩ and k was 0.4, the sensitivity reduced from 0.9 to 0.25. Similarly, when k was 0.5 and skin resistance (REC) was increased from 2 to 10 kΩ and, the sensitivity reduced from 0.95 to 0.35. Fig. 2d shows how variation in skin capacitance (CCM) affects sensitivity. The sensitivity was studied at various skin capacitances (CCM) while the coupling coefficient was k = 0.4. The sensitivity increased from 0.20 to 0.28 when skin capacitance changed from 8 to 12 pF. The sensitivity increased from 0.27 to 0.4 when skin capacitance (CCM) increased from 8 to 12 pF, and coupling coefficient was maintained at k = 0.5. The data show that sensitivity gradually increases by increasing the skin capacitance (CCM) and decreases with increasing skin resistance (REC).
Finite element analysis (FEA) was conducted to study the current distribution on human skin with the tumor radius of 2.4 mm in stage 235,36. Figure 2e shows the current density when the distance between electrodes was 1 cm. Similarly, the current density was measured by placement of the electrodes on the tumor and distance between the electrodes was set to 2 mm. The current distribution of different sizes and distances between the tumor and electrodes are shown in (Supplementary Figs. 3–7). The data show the current density is higher when the electrodes touch the tumor as compared to the electrodes placed far from the tumor. FEA was also performed to further evaluate the current distribution in benign and malignant tumors. Figure 2f shows the current density significantly varies between benign and malignant tumors where the current is higher in malignant tumors. According to Warburg effect, the glycolysis rate is high in cancerous cell that leads to increase in glucose uptake and lactic acid production37. As a result, the intracellular and extracellular pH values increase significantly in cancerous cells compared to normal cells and generate greater ion concentrations. The elevated ion concentration increases the conductivity and permittivity in cancerous cells. Therefore, the conductivity of the malignant tissue is higher than non-malignant cells38,39,40, resulting in a higher current density in the malignant tumors. In addition, studies showed that tumor tissues exhibit elevated temperatures, which can impact the impedance by modifying the dielectric properties of cell membranes and enhancing ionic mobility41. It has been reported that for every 1 °C increase in temperature, tissue conductivity can increase by approximately 2%, leading to a reduction in electrical impedance at some frequencies42. These temperature fluctuations introduce variations in impedance and are inherently part of the physiological differences between normal and cancerous tissues and are not caused by external environmental factors. We also evaluated the environmental factors on the patch sensitivity. We conducted experiments on free space and skin under varying humidity and temperature, as shown in Supplementary Fig. 15. The results showed consistent patch performance despite the environmental changes.
Device characterization and effect of electrodes on impedance measurement
The wireless battery-free, non-invasive patch was characterized by measuring power reflection using a vector network analyzer (VNA) to evaluate the impedance of the skin. The power reflection was measured when the relative position between the reader and the patch was changed as shown in Fig. 3a. The patch was fixed at 3 mm from the reader module and the maximum power reflection on the network analyzer was measured as −12.5 dB. When distance between the reader module and the patch increased from 3 to 7 mm, the power reflection was as low as −30 dB. The reason for more reflection at closer distance is due to strong coupling between the coils. The splitting in resonance from the coupled reader and patch module effectively reduces the power transmission to the patch. Further increasing the distance from 7 to 11 mm increases the power reflection and reduces the power delivery (Eq. 3 and Fig. 2a). Within the 3 mm to 9 mm separation range, power transfer efficiency is greater than 90% (Supplementary Fig. 17), showing the optimal operating range for the device. The power from the VNA coupled to the reader module was 0 dBm (1 mW).
a Power reflection of the impedance measurement system when the reader and the patch are at different distances. b Measurement of human skin bioimpedance from power reflection for dry and wet electrodes. c Dependance of the power reflection on frequency when human skin model has different resistance. d Dependance of the power reflection on frequency when human skin model has different capacitance. e, f Comparison of the quality factor by varying the values of resistance and capacitance of the human skin circuit model.
The impedance measurement was performed on a human subject with a dry electrode where the patch was directly placed on skin without any interface. The experiment was repeated on the same part of skin by applying a conductive gel (Spectra 360 electrode gel) to the electrodes as shown in Fig. 3b. The power reflection and resonant frequency were changed significantly because of the change in the impedance of the interface for dry and wet electrodes. The minimum power reflection was −6 dB with the gel in the interface whereas the minimum power reflection of −7 dB was recorded in the case of dry electrodes. Additionally, the resonant frequency was reduced to 4.85 MHz for the wet electrode where the resonance frequency with dry electrodes increased to 5.01 MHz. The changes in the reflection coefficient S11 and resonant frequency are due to unstable interface of dry electrodes, which results in variable contact impedance (Supplementary Figs. 10-11). Additionally, a significant variation may exist in electrode-skin impedance due to skin moisture and unwanted gaps in the interface43. Application of proper gels can enhance the quality of measurement and improve accuracy because conductive gel provides high-fidelity electrical contact with the skin. In addition, the conductive gel at the skin-electrode interface maintains stable contact and prevents disconnection caused by bending, stretching and deformation. To evaluate this, we experimentally assessed the patch’s signal stability under both flat and bent conditions (up to 15°), performing 10 repeated trials per condition (Supplementary Fig. 18). The reflection coefficient (S₁₁) showed mean values of –14.82 dB (unbent) and –14.80 dB (bent), with corresponding standard deviations of 0.02 and 0.01, respectively, indicating minimal impact of bending on patch performance. Moreover, previous studies demonstrated that ultrathin, flexible bioelectronic patches fabricated on soft elastomeric substrates can maintain stable electrical performance under various forms of mechanical strain, including bending and stretching44,45,46. These systems typically exhibit minimal changes in signal quality even after repeated deformation cycles, highlighting their mechanical reliability during normal human activity47.
We conducted experiments and evaluated the patch on an electrical circuit, representing electrical model of skin tissues and tumors. The impedance of tumor varies with the frequency and the size of the tumor, generally being lower than the normal tissue. Cancer cells have higher sodium concentration and water content48, resulting in high capacitance and low resistance than normal cells49. The impedance of the cancer cell decreases as cancer progresses ranging from 1 kΩ to 100 Ω depending on the stage50. Power reflection and resonant frequency were measured at different load resistances REC (1 kΩ, 2 kΩ, and 3 kΩ) and capacitances CCM (7 pF, 10 pF, and 12 pF) to simulate skin impedances as illustrated in Fig. 3c, d. The results show similar trends in the power reflection (S11) and resonant frequency (⍵r) based on the variations in load resistance (REC) and capacitance (CCM). As the resistance in the circuit increased from 1 to 3 kΩ by maintaining capacitance constant, S11 shifted from −2.0 to −6.2 dB as shown in Fig. 3c. The results show that the reflected impedance (Zr) is reduced at higher resistance of the load, which reduces the overall impedance (Zin) of the circuit. Similarly, Fig. 3d shows the inverse relation between the skin capacitance and resonant frequency (⍵r). The resonant frequency (⍵r) is reduced at higher skin capacitance. This agrees with Eqs. 1–3, that power reflection (S11) and resonant frequency (⍵r) are sensitive to the changes in the impedance of the load (ZL). The data shows that more energy is transferred at higher resistance REC and capacitance CCM of the load. Quality factor (QpL) of the patch along with the bioimpedance was studied in Fig. 3e and f and is defined as51:
Figure 3e, f demonstrated that quality factor (QpL) of the patch attached to skin increases by increasing the resistance and capacitance of the skin. Figure 3e showed that the quality factor (QpL) changes from 2.75 to 2.81 when the capacitance changes from 8 to 12 pF and the resistance is 1 kΩ. Figure 3f shows that quality factor (QpL) changes from 3.5 to 27.5 when the resistance changed from 1 kΩ to 10 kΩ. Therefore, bioimpedance (ZL) affects the quality factor of the measurement system and is more sensitive to changes in resistance than capacitance of the load.
Human subject study for wireless measurement of bioimpedance of skin
Ten human subjects participated in this study to measure the impedance of pigmented lesions (e.g., moles) versus healthy skin tissues in the absence of lesions. A reader module was connected to a VNA, and a patch was attached to human skin using a medical tape. The electrodes on patch established an electrical connection between the skin and the patch. The distance between the electrodes was fixed at 1 cm, and the diameter of the electrodes was 1 mm as shown in Fig. 4a. The reader and the patch were positioned 3 mm from each other. The flowchart of the impedance measurement system is also shown in Fig. 4a. First, the VNA generates the signal that powers the coils in the reader module and the patch is inductively coupled to the reader to induce the voltage into the patch. The data were collected using the VNA to determine the S-parameters. The reflection spectrum from VNA was obtained for the measurement of the impedance of each subject’s skin and lesions. The magnitude and phase responses of reflection coefficient S11 are shown in Fig. 4b, c and Supplementary Fig. 8. The experiment was conducted on a mole and on the skin near the mole. The mole showed the minimum magnitude of reflection coefficient S11 was −6.5 dB where the skin in 2 cm from the mole had a magnitude of −12.2 dB. The phase difference between the mole and skin was 19 degrees. The phase angle is associated with the capacitance produced by cell membrane and interface with the tissue. Results from the phase angle and magnitude of S11 indicate that moles have different dielectric constants and permeabilities compared to normal tissue on skin. Furthermore, the quality factor was significantly higher for healthy skin with a value of 24.7 with a bandwidth of 0.16 MHz as compared to 6.3 for mole with a bandwidth of 0.66 MHz. In addition, various areas of the body were evaluated on a male subject to study the change in the reflection coefficients. It was noted that distinct body regions exhibited different reflection coefficient (S11) and frequency responses as shown in Fig. 4d. The dissimilarities in tissue characteristics such as skin composition, thickness, moisture content, and underlying tissues cause variations in S11 parameter and frequency response.
a Bioimpedance measurement of mole and skin using the patch on various parts of body, micrograph of a pigmented lesions and location of electrodes, and a flowchart of measuring the bioimpedance of moles and healthy skin. b, c Magnitude and phase of reflection coefficient measured by the patch on mole and on healthy skin. d Change in reflection coefficients and resonance frequencies at different locations on human skin. e, f Change in the capacitance and resistance was measured on mole and on the skin for ten subjects. g Comparison of Impedance measurement on different subjects with the Nevisense device.
The experiment was repeated for 15 times to verify the repeatability of the results. The outcome consistently demonstrated a standard deviation of 0.8 for mole and 1.05 for healthy skin as presented in Supplementary Figs. 9, 16. Supplementary Eq. 7 demonstrated that 95% of the results fall within two standard deviations of the mean. The reflection coefficient S11 on moles and healthy skin were measured from ten distinct subjects. Capacitance (CCM) and resistance (REC) with the measured S11 from participants were calculated using resonance frequency (Eq. 1) and quality factor (Eq. 7) as shown in Fig. 4e, f. Results from 10 subjects consistently demonstrated that the device recorded a higher mean capacitance (7.3 pF) and lower mean resistance (4.5 kΩ) over moles, in contrast to adjacent healthy skin, which exhibited a lower mean capacitance (3.8 pF) and higher mean resistance (8.3 kΩ). These findings are aligned with previous studies on tissue impedances52,53. A commercial EIS device (Nevisense) was used to measure the impedance of the moles and healthy skin for comparison to the results obtained from our patch. The results in Fig. 3g from three human subjects also consistently demonstrated that impedance of moles is lower than normal skin tissues. Nevisense and wearable patch operate at different frequency ranges and have different electrode configurations which resulted in differences in the impedance reading.
Discussion
We presented a wearable, chip-less, battery-free, wireless patch for measuring the bioimpedance of pigmented lesions and healthy skin tissues. The patch will be instrumental for detecting abnormal impedances on skin for early detection of melanoma risks. In addition, the skin impedances obtained from wearable patches will be complementary information that enhances diagnostic accuracy and assists with clinical decision making, particularly when integrated with conventional optical approaches. The patch is flexible and skin-conformal, which represents an easy-to-use and comfortable device for evaluating the impedance of decolored regions of skin and lesions. The patch will enable monitoring of the lesions by patients and primary-care providers outside of specialized clinical settings. Our results indicated that the wearable patch in conjunction with the reader module can measure the bioimpedances in wireless mode through magnetic induction. In addition, the results showed that moles have different capacitance (CCM) and resistance (REC) compared to healthy skin tissues. Furthermore, the impedance of healthy skin tissue is higher than pigmented lesions in our pilot human study. The wearable patch provided reliable and repeatable results in measurement of cutaneous impedance on participants where 95% of the results fall within the two standard deviations of the mean for 15 repeated trials (Supplementary Eq. 7 and Figs. 9 and 16). We tested the patch on 10 human subjects as proof of the concept that the wearable patch can detect impedance change from pigmented lesions and healthy skin.
The RF-powered patch achieves optimal energy transfer within a 10 mm range that constrains continuous monitoring applications. However, the system is very effective for point-of-care applications and periodic assessments of lesions. The battery-free architecture eliminates rigid components, improving comfort and reducing the risk of adverse skin reactions, particularly important for abnormal or delicate tissue, such as melanoma. Furthermore, our experiments showed that the standard deviation for power reflection is less than 2% when the device is bent up to 15°, showing the device is functional under minor bending from body motions. This is aligned with previous studies that showed flexible bioelectronic patches can maintain stable electrical performance under various forms of mechanical strain including bending and stretching44,45,46,47.
As part of future work, conductive hydrogel will be integrated into electrodes to enhance sensor performance, signal quality, and ease of use. In addition, we plan to conduct clinical evaluations of the wearable patch for in vivo impedance measurements in melanoma lesions. This will assist in differentiating between healthy skin tissues and malignant tumors for identification of melanoma risks in the early stage.
Methods
Device fabrication
The fabrication of a reader module and a patch is shown in Supplementary Figs. 1, 2. The reader module is constructed using a capacitor and pre-made inductor coil consisting of 40 turns on a rigid board. The patch consists of a 40-turn inductor and a capacitor. The circuit on the patch was incorporated into a double-layered printed circuit board (PCB) on a 100-µm thick polyimide substrate. The inductor dimension on the reader was 53 mm × 44 mm. A surface mounted capacitor, measuring 1 mm × 1 mm × 0.5 mm was soldered to the flexible patch. A copper tape is used to establish a connection between the outermost turn of the coil and the capacitor. The dimensions of the patch were approximately 1 mm × 25 mm × 25 mm. Electrodes were used to establish a connection between patch and tissue for impedance measurement.
Device characterization
Wireless detection was accomplished through Agilent network analyzer through a 12-inch SMA (Subminiature version A) cable. One port of the vector network analyzer (E5063A, Keysight Technologies, USA) was connected to the reader module and distance between the reader and the patch was adjusted to characterize the separation range. For the characterization of the range of the impedance, the resistance and capacitance of the bioimpedance circuit varied while maintaining the constant distance. The performance of the device was also tested with and without conductive gel in the interface of skin and electrodes on the patch.
Human subject study
Human subject study was reviewed and approved by Institutional Review Board (IRB) of Northern Illinois University (HS23-001). Subject participation was completely voluntary, and consent was obtained from the participants before starting data collection. No complaints or reports of pain in the site of measurement were received from the subjects during and after the experiment.
Statistical analysis
Statistical analysis was performed in R Studio. Prior to statistical comparison, the Shapiro-Wilk test was performed to assess the normality of the capacitance and resistance data across the 10 subjects. Both capacitance and resistance measurements for mole and healthy skin sites were found to be normally distributed with a P-value of 0.5 and 0.1 (P > 0.05). Subsequently, paired t-tests were conducted to evaluate the differences in capacitance and resistance between mole and healthy skin regions. The result showed a statistically significant difference with a P-value of 0.01 and 0.003 (P < 0.05).
Device biocompatibility
The substrate, polyimide, is biocompatible, and the electrical components in the patch are Restriction of Hazardous Substances (RoHS)-compliant”.
Finite Element Analysis of Human Skin Model
Electrical current density was simulated using COMSOL Multiphysics software within a human skin model. We established the material properties and geometry of the skin model by making layers of the skin—stratum corneum, epidermis, dermis, and by defining the dimensions and electrical characteristics such as permittivity and conductivity. The ‘AC/DC’ module was utilized to configure interfaces and boundaries for current flow and ensure accuracy by generating a well-suited mesh. We conducted simulations by establishing solver parameters and physics equations related to current flow to compute the distribution of current density across the skin model. Results are analyzed using COMSOL’s visualization tools by creating the plots and displaying the spatial distribution of current density in Supplementary Figs. 3–7. The effects of tumor on current density were evaluated by changing the distance between the electrodes and the shape of tumor on human skin. Spherical and circular shaped tumor models were used for the comparison. These representations demonstrated how electrical currents disperse within the skin model under various conditions which are important for the detection of melanoma.
Measurement of human skin bioimpedances
Our initial approach was to investigate the interaction between a reader, a patch, and various human skin tissues, focusing on the detection of the S11 parameter. The setup comprised of a reader module, a specifically designed patch, and a vector network analyzer (VNA). Various participants volunteered to measure the bioimpedance of their skin and pigmented lesions. The patch and reader were fixed at a certain distance to maintain stability throughout the measurements. The setup and the VNA were calibrated with various loads with known impedances before starting the experiments. The patch was placed on the skin of each participant to connect the tissue electrically and obtain S11. We conducted experiments to detect and record the S11 parameter, change in the reflection coefficient, as the reader interacted with the patch on various skin tissues. Magnitude and phase of the reflection coefficient S11 were evaluated on lesions, near lesions and on various parts of skin for the measurement of human skin impedance as shown in Supplementary Fig. 8. The results of the overall input impedance (Zin) are shown in Supplementary Fig. 14.
Measurement for repeatability
To ensure the consistency of our results, we conducted multiple repetitions of the experiment on every subject to find power reflection coefficient S11. This process allowed us to verify the reliability of our findings and enhance the repeatability of our conclusions. By continuously repeating the experiment, we aim to minimize any potential errors or fluctuations in the data. Z score was used to find consistency and repeatability in the measurement. The equation is shown in Supplementary Eq. 7. Results of the measurements are shown in Supplementary Fig. 9. Calculations from Z score equation demonstrated that 95% of the results fall within the two standard deviations of the mean.
Dry and wet electrodes
Two different non-invasive surface electrodes are available: gel electrode and dry electrode. The impedance between these electrodes and the skin strongly impacts the quality of the acquired bioelectrical signals. An experiment was conducted to observe the change in the voltage through skin circuit (ZL). We observed high voltage with gel electrodes relative to dry electrodes because of the additional impedance due to gap with dry electrodes as shown in Supplementary Figs. 10, 11. Therefore, conduction is higher through gel than dry electrodes.
Data availability
All data are available in the main text or the supplementary materials.
References
Cazzato, G. Histopathological Diagnosis of Malignant Melanoma at the Dawn of 2023: Knowledge Gained and New Challenges. Dermatopathology 10, 91–92 (2023).
Waseh, S. & Lee, J. B. Advances in melanoma: epidemiology, diagnosis, and prognosis. Front Med. (Lausanne) 10, 1268479 (2023).
Dinnes, J. et al. Visual inspection for diagnosing cutaneous melanoma in adults. Cochrane Database Syst. Rev. 2018, CD013194 (2018).
Deda, L. C., Goldberg, R. H., Jamerson, T. A., Lee, I. & Tejasvi, T. Dermoscopy practice guidelines for use in telemedicine. NPJ Digit Med. 5, 55 (2022).
Akinrinade, O. & Du, C. Skin cancer detection using deep machine learning techniques. Intell. Based Med. 11, 100191 (2025).
Ozdemir, B. & Pacal, I. A robust deep learning framework for multiclass skin cancer classification. Sci. Rep. 15, 4938 (2025).
Kreouzi, M. et al. Deep Learning for Melanoma Detection: A Deep Learning Approach to Differentiating Malignant Melanoma from Benign Melanocytic Nevi. Cancers (Basel) 17, 28 (2024).
Cukras, A. R. On the Comparison of Diagnosis and Management of Melanoma Between Dermatologists and MelaFind. JAMA Dermatol 149, 622 (2013).
Venkatesh, K. P., Kadakia, K. T. & Gilbert, S. Learnings from the first AI-enabled skin cancer device for primary care authorized by FDA. NPJ Digit Med. 7, 156 (2024).
Dean, D. A., Ramanathan, T., Machado, D. & Sundararajan, R. Electrical impedance spectroscopy study of biological tissues. J. Electrostat 66, 165–177 (2008).
Heimburg, T. The Capacitance and Electromechanical Coupling of Lipid Membranes Close to Transitions: The Effect of Electrostriction. Biophys. J. 103, 918–929 (2012).
Baidillah, M. R. et al. Electrical impedance spectroscopy for skin layer assessment: A scoping review of electrode design, measurement methods, and post-processing techniques. Measurement 226, 114111 (2024).
Khalil, S., Mohktar, M. & Ibrahim, F. The Theory and Fundamentals of Bioimpedance Analysis in Clinical Status Monitoring and Diagnosis of Diseases. Sensors 14, 10895–10928 (2014).
Litchman, G., Teplitz, R., Svoboda, R. M. & Del Rosso, J. Q. Increased Uniformity in Diagnostic Accuracy of Pigmented Lesions Using Electrical Impedance Spectroscopy Information. J. Clin. Aesthet. Dermatol 14, 35–36 (2021).
Rocha, L. et al. Analysis of an electrical impedance spectroscopy system in short-term digital dermoscopy imaging of melanocytic lesions. Br. J. Dermatol. 177, 1432–1438 (2017).
Zakria, D. et al. Electrical impedance spectroscopy significantly enhances correct biopsy choice for pigmented skin lesions beyond clinical evaluation and dermoscopy. Melanoma Res. 33, 80–83 (2023).
Owji, S. et al. Diagnostic Efficacy of Electrical Impedance Spectroscopy Versus Dermoscopy for Pigmented Skin Lesions: A Pilot Study. SKIN J. Cutan. Med. 6, 210–216 (2022).
Jung, J. M. et al. Emerging Minimally Invasive Technologies for the Detection of Skin Cancer. J. Pers. Med. 11, 951 (2021).
Moqadam, S. M. et al. Cancer detection based on electrical impedance spectroscopy: A clinical study. J. Electr. Bioimpedance 9, 17–23 (2018).
Dendy, P. P. & Meldrum, R. A. CHARACTERISTICS OF THE MALIGNANT CELL AND THE CELL CYCLE IN TUMOURS. In Placenta 176–214 https://doi.org/10.1016/B978-0-08-024435-8.50027-4 (Elsevier, 1979).
Malvehy, J. et al. Clinical performance of the Nevisense system in cutaneous melanoma detection: an international, multicentre, prospective and blinded clinical trial on efficacy and safety. Br. J. Dermatol. 171, 1099–1107 (2014).
Gómez Cortés, J. C. et al. Modular and Portable System Design for 3D Imaging of Breast Tumors Using Electrical Impedance Tomography. Sensors 24, 6370 (2024).
Lee, H. et al. Wearable Graphene Tattoo Impedance Tomography for Skin Lesion Differentiation. In 2023 IEEE Biomedical Circuits and Systems Conference (BioCAS) 1–5 https://doi.org/10.1109/BioCAS58349.2023.10388782 (IEEE, 2023).
Yang, P. et al. A wearable triboelectric impedance tomography system for noninvasive and dynamic imaging of biological tissues. Sci. Adv. 10, eadr9139 (2024).
Gilad, O., Horesh, L. & Holder, D. S. Design of electrodes and current limits for low frequency electrical impedance tomography of the brain. Med Biol. Eng. Comput 45, 621–633 (2007).
Gómez-Cortés, J. C. et al. Electrical Impedance Tomography Technical Contributions for Detection and 3D Geometric Localization of Breast Tumors: A Systematic Review. Micromachines (Basel) 13, 496 (2022).
Keung, E. Z. & Gershenwald, J. E. The eighth edition American Joint Committee on Cancer (AJCC) melanoma staging system: implications for melanoma treatment and care. Expert Rev. Anticancer Ther. 18, 775–784 (2018).
Tsai, B., Xue, H., Birgersson, E., Ollmar, S. & Birgersson, U. Dielectrical properties of living epidermis and dermis in the frequency range from 1 kHz to 1 MHz. J. Electr. Bioimpedance 10, 14–23 (2019).
Ertsgaard, C. T., Kim, M., Choi, J. & Oh, S.-H. Wireless dielectrophoresis trapping and remote impedance sensing via resonant wireless power transfer. Nat. Commun. 14, 103 (2023).
Mariotti, C. et al. Dual-frequency antennas embedded into the floor for efficient RF “energy evaporation”. In 2015 IEEE 65th Electronic Components and Technology Conference (ECTC) 2066–2070 https://doi.org/10.1109/ECTC.2015.7159887 (IEEE, 2015).
Lv, X. et al. Experimental demonstration of broadband impedance matching using coupled electromagnetic resonators. Sci. Rep. 10, 7437 (2020).
Tang, W. et al. Optimization of magnetic coupling mechanism of dynamic wireless power transfer based on NSGA-II algorithm. Sci. Rep. 14, 5121 (2024).
Nikitin, P. V. et al. Power reflection coefficient analysis for complex impedances in RFID tag design. IEEE Trans. Micro. Theory Tech. 53, 2721–2725 (2005).
Bora, D. J. & Dasgupta, R. Various skin impedance models based on physiological stratification. IET Syst. Biol. 14, 147–159 (2020).
Gershenwald, J. E. et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 67, 472–492 (2017).
Amin, M. B. et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 67, 93–99 (2017).
Warburg, O. On the Origin of Cancer Cells. Science (1979) 123, 309–314 (1956).
Taghian, T., Narmoneva, D. A. & Kogan, A. B. Modulation of cell function by electric field: a high-resolution analysis. J. R. Soc. Interface 12, 20150153 (2015).
Goodman, E. M., Greenebaum, B. & Marron, M. T. Effects of Electromagnetic Fields on Molecules and Cells. In 279–338 https://doi.org/10.1016/S0074-7696(08)62489-4 (1995).
Fahmy, H. M. et al. Dielectric spectroscopy signature for cancer diagnosis: A review. Micro. Opt. Technol. Lett. 62, 3739–3753 (2020).
Sharma, R. & Malviya, R. Modifying the electrical, optical, and magnetic properties of cancer cells: A comprehensive approach for cancer management. Med. Adv. 2, 3–19 (2024).
Qian, P. C. et al. Optimizing Impedance Change Measurement During Radiofrequency Ablation Enables More Accurate Characterization of Lesion Formation. JACC Clin. Electrophysiol. 7, 471–481 (2021).
Kaufmann, S., Ardelt, G. & Ryschka, M. Measurements of Electrode Skin Impedances using Carbon Rubber Electrodes – First Results. J. Phys. Conf. Ser. 434, 012020 (2013).
Choi, S., Lee, H., Ghaffari, R., Hyeon, T. & Kim, D. Recent Advances in Flexible and Stretchable Bio-Electronic Devices Integrated with Nanomaterials. Adv. Mater. 28, 4203–4218 (2016).
Rogers, J. A., Someya, T. & Huang, Y. Materials and Mechanics for Stretchable Electronics. Science (1979) 327, 1603–1607 (2010).
Xu, S. et al. Soft Microfluidic Assemblies of Sensors, Circuits, and Radios for the Skin. Science (1979) 344, 70–74 (2014).
Kim, D.-H. et al. Epidermal Electronics. Science (1979) 333, 838–843 (2011).
Lee, R. et al. Dielectric imaging for differentiation between cancer and inflammation in vivo. Sci. Rep. 7, 13137 (2017).
Yang, O. C. Y. & Loh, S.-H. Acidic Stress Triggers Sodium-Coupled Bicarbonate Transport and Promotes Survival in A375 Human Melanoma Cells. Sci. Rep. 9, 6858 (2019).
Hedayatipour, A., Aslanzadeh, S. & McFarlane, N. CMOS based whole cell impedance sensing: Challenges and future outlook. Biosens. Bioelectron. 143, 111600 (2019).
Coleman, C. Introduction to Radio Frequency Engineering.
Chinitz, J., Michaud, G. & Stephenson, K. Impedance-guided Radiofrequency Ablation: Using Impedance to Improve Ablation Outcomes. J. Innov. Card. Rhythm Manag 8, 2868–2873 (2017).
Ugwaha, J. et al. Ex-Vivo Detection of Breast Cancer with a Bio-Impedance Sensor. J. Cancer Treat. Diagn. 5, 18–22 (2021).
Liebich, C., Bartsch, J. N., Schubert, I., von Bruehl, M.-L. & Sander, C. Electrical Impedance Spectroscopy Improves Skin Cancer Detection and Reduces the Number of Biopsies. Dermato 2, 21–29 (2022).
Acknowledgements
This work was supported by the National Institute on Deafness and other Communication Disorders (NIDCD), National Institutes of Health (NIH) under award number R21DC018894, Atrium Health Wake Forest Baptist Comprehensive Cancer Center, Rowan University-College of Engineering and Northern Illinois University Research and Artistry Award. The authors would like to thank Priyanka Kumar and Daniel Massat for their preliminary studies on wireless impedance measurements.
Author information
Authors and Affiliations
Contributions
Conceptualization: M.J.M.; Methodology: H.I., E.R., E.L., M.J.M.; Investigation: H.I., E.R., M.T., M.J.M.; Visualization: E.R., H.I., E.L., M.J.M.; Funding acquisition: M.J.M.; Project administration: M.J.M., M.T.; Supervision: M.J.M.; Writing—original draft: H.I., E.R., M.J.M.; Writing—review & editing: H.I., E.L., M.T., M.J.M.
Corresponding author
Ethics declarations
Competing interests
M.J.M. has an international patent cooperation treaty (PCT) application on wireless bioimpedance measurement with wearable patches.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Imtiaz, H., Ray, E., Lim, E. et al. Wearable battery-free chip-less patch for bioimpedance measurement of cutaneous lesions. npj Biomed. Innov. 2, 32 (2025). https://doi.org/10.1038/s44385-025-00037-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s44385-025-00037-7