Abstract
Organic nitrogen (ON) constitutes an important fraction of nitrogenous aerosols, impacting ecosystems, secondary organic aerosol formation, and biotoxicity. However, knowledge of aerosol ON levels and sources has remained largely unexplored. This study uses receptor modeling to apportion aerosol ON, based on bihourly measurements of ON and source-specific molecular and elemental markers at suburban Hong Kong during winter of 2020–2021. Aerosol ON averaged at 0.26 ± 0.09 μgN/m3, with comparable contributions from primary (54.0%) and secondary sources (46.0%). Bihourly resolution enabled observation of diurnal-scale dynamics of ON sources and formation chemistry, capturing short-term ON episodes. Biomass burning, α-pinene-related SOA formation, and sulfate-rich factors were dominant contributors during ON episodes. Fractional abundance of ON increased with humidity and temperature, reflecting the combined consequence on the formation of ON and gas-particle partitioning of inorganic nitrogen. Nighttime ON notably increased under high NOx levels, highlighting the role of NO3 radical chemistry in secondary ON formation.
Similar content being viewed by others
Introduction
Aerosol organic nitrogen (ON) is an important fraction of the atmospheric nitrogenous aerosols1,2. As recently estimated by Yu et al.1, nitrogenous organic aerosol (OAN) typically represents 37–50% of organic aerosols (OA) in the atmospheres of significant urban influence. In addition to its vital role in the biogeochemical cycling3,4, certain ON species are important brown carbon constituents5,6,7, and closely linked to new particle formation8 as well as secondary organic aerosol (SOA) formation9, and can impact regional air quality and global climate. Besides, many ON constituents such as nitro-aromatic compounds (NACs) and nitrogen-heterocyclic compounds have been demonstrated as potent carcinogens leading to mutagenesis and genotoxicity10,11, thus public health concerns.
OAN is highly complex in its chemical composition, reflecting its diverse sources1,2. These sources include natural origins, such as marine, dust, vegetation, and microorganisms, as well as anthropogenic activities such as biomass burning, coal combustion, industrial processes, agricultural activities, and animal husbandry, etc.2,12,13,14,15,16,17. In addition, secondary formation pathways, such as photochemical and dark reactions during transport, play significant roles in the generation of ON species18,19,20,21,22. Over the past several decades, extensive efforts have been made to investigate the sources, key controlling factors, and formation pathways of aerosol water-soluble ON across various environments, from urban areas23,24, rural areas23,24,25, forests26,27, coastal sites28,29, marine sites30,31,32,33, to high-elevation locations34. Past aerosol ON studies have predominantly focused on the water-soluble ON fraction (i.e., WSON), while the water-insoluble ON (WION) has received limited attention due to the lack of a direct quantification method35. Only a small number of studies have estimated WION by calculating the difference between total nitrogen (TN) and water-soluble TN (WSTN), and have reported more abundant WION than WSON31,34,36,37. It is important to note, however, that previous measurements of WSON and WION were associated with considerable uncertainties inherent in the difference approach, and that TN quantification has been constrained by relatively high detection limits (~0.7 μgN) associated with the use of elemental analyzers35,38.
Previous source studies of aerosol ON have primarily relied on offline filter-based measurements and were mostly limited to WSON. The temporal resolution of these datasets, typically daily or sub-daily, is inadequate for capturing diurnal-scale variations in source emissions, secondary formation processes (e.g., daytime photochemistry and nighttime nitrate radical chemistry), and rapid changes in ambient conditions such as temperature, relative humidity, and aerosol acidity39. Filter-based studies have reported varying diurnal patterns in ON levels. For example, Rastogi et al.40 observed consistently higher nighttime WSON and N:C ratios compared to daytime levels in the southeastern United States, attributing the nocturnal increase to SOA formation through dark reactions and the influence of a shallow nighttime boundary layer. A similar enrichment of nocturnal WSON was observed at a suburban forest site in Japan, likely driven by aqueous reactions under conditions of increased aerosol liquid water22. In contrast, stable isotope analysis of filter samples from Mt. Tai in China revealed significantly higher daytime ON concentrations, primarily ascribed to the effects of mountain-valley breezes and changes in boundary layer height41. Despite these observations, the coarse time resolution of offline measurements has precluded detailed investigation of the diurnal variations in source origins and formation mechanisms of atmospheric ON. This limitation highlights the necessity of online measurements of aerosol ON and source-specific tracers with high time resolution.
In recent decades, online mass spectrometry techniques such as aerosol mass spectrometer (AMS) and chemical ionization mass spectrometer (CIMS) have been employed to characterize the chemical composition of aerosol ON. These instruments offer high-resolution chemical speciation, and when combined with positive matrix factorization (PMF) or correlation analysis, they can provide insights into potential sources and transformation processes of ON. However, most studies to date have focused on a limited few groups of ON compounds, such as organic nitrates and NACs, rather than achieving mass closure of bulk ON aerosols9,42,43,44,45. To our knowledge, the study by Xu et al.21 is the only one that quantified bulk aerosol ON using AMS. Their findings indicate that secondary formation processes dominated ON during three seasons in urban Beijing, except for winter when biomass burning emerged as a more important ON source. Nevertheless, quantifying ON using AMS is associated with significant uncertainties. Although AMS can determine ON in the form of CxHyNp+, CxHyOzNp+, and CxHyOzNpSq+ ion fragments, separation of ON and IN contributions within the NHx+ and NOx+ families is difficult to achieve. This drawback can lead to the underestimation of ON by as much as ~42%21. Similarly, CIMS is constrained by its selective sensitivity of the reagent ion and the lack of calibration standards for many ON compounds, both of which hinder the accurate characterization of bulk ON. In light of these limitations inherent to current online mass spectrometry techniques, there is a clear need to employ complementary quantification methods that can achieve robust mass closure and thereby attain a more comprehensive understanding of ON sources.
Our research team has recently developed an analyzer system that enables the simultaneous determination of IN and ON in aerosol samples and we have demonstrated its applications for both offline filter analysis35,46 and online monitoring47,48. This analyzer is based on programmed thermal evolution up to 800 °C and catalytic conversion of nitrogenous aerosols to NO, followed by chemiluminescent detection. This approach offers a distinct advantage by capturing the complete mass of ON, including both WSON and WION. Our previous work utilizing online ON measurement focused mainly on investigating the episodical variations of ON concentration and the driving factors at urban and suburban sites. In the present study, we utilized total ON measurements, complemented by concurrent measurements of molecular and elemental source tracers. By incorporating a comprehensive suite of online measurements of organic and inorganic tracers with hourly or bihourly time resolution, we were able to identify the major sources of aerosol ON, quantify their contributions, and gain a quantitative understanding of the dynamic evolution processes of ON at a diurnal scale. This work provides one of the few first online ON measurements and its source apportionment at a bihourly time resolution47,48. Such field observation-based insights are essential for enabling quantitative assessment of the environmental and health impacts of OAN as well as informing projections of how OAN levels and sources may respond to future changes in climate.
Results
Abundances of IN and ON in PM2.5
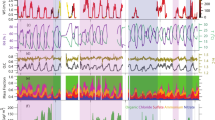
Figure 1 presents the time series of the nitrogenous aerosol concentrations at HKUST during the field campaign, along with the meteorological parameters, gaseous pollutants, and PM2.5 concentration. A statistical summary of the measured parameters is provided in Table S3. Over the measurement period, aerosol TN concentrations ranged from 0.30 to 6.33 μgN/m3, with an average value of 2.01 ± 1.04 μgN/m3. The average IN and ON concentrations were 1.76 ± 0.99 μgN/m3 and 0.26 ± 0.09 μgN/m3, respectively. Our recent work1 derived a range of values for the OAN-to-ON ratio (the factor converts ON mass to nitrogenous OA mass) using Monte Carlo simulations constrained by abundance ranges of subgroups of ON molecules found in the literature. The simulations generated a population of this ratio quantity, which followed a log-normal distribution having the mean value of 7.16 and a 95% confidence interval of [6.45,7.78]. By applying the mean value to the data in our current study, we estimate the campaign-average nitrogenous OA mass was 1.9 ± 0.6 μg/m3. This corresponded to ~25% of OA (assuming an OM-to-OC ratio of 1.8) and 11% of PM2.5 mass, highlighting the significant contribution of OAN to OA and PM2.5 pollution.
The ON abundance at our site was similar to that measured at a plateau forest region of China (0.27 ± 0.13 μgN/m3)34, but was significantly lower than the annual average WSON abundance reported at a background site (0.71 ± 0.43 μgN/m3), a rural site surrounded by forests (0.73 ± 0.57 μgN/m3), and an urban site (0.50 ± 0.45 μgN/m3) in the PRD region of China24. Note that the ON and WSON concentrations reported in the two above-mentioned studies were determined using the difference method, which introduces uncertainties that can be significant, especially in cases where the IN fraction in TN is large.
The hourly fractional contribution of ON to TN at our site showed significant variation, ranging from 5.6 to 63.6%. This indicates that ON could sporadically reach elevated levels to become a dominant part of aerosol TN. Figure S2 presents the frequency distribution of the fractional contribution of ON, which approximately follows a log-normal distribution pattern, with a mode value of 12.8% and a mean value of 12.9%. In a previous study, our research team quantified the bulk ON aerosols in daily filter samples at an urban site in Hong Kong using the offline IN & ON analyzer system35. The ON concentration measured in the current study was approximately one-third of the wintertime ON concentration at that urban site (0.26 vs. 0.84 μgN/m3). Additionally, the proportion of ON in TN was lower in the current study (12.9% vs. 25.1%). These differences likely reflect the diverse sources of nitrogenous aerosols in different environments within the same megacity. The discrepant sampling rates of aerosols in the online and offline ON measurements may also contribute to the observed differences in ON concentration between this study and the previous study.
Figure 2a indicates that the fractional contribution of ON to TN was higher at lower TN loadings. As TN increased from 0.25 to 6.0 μgN/m3, the fraction of ON decreased from 37 to 8%. Higher TN levels were generally linked to larger proportions of IN (NO3−-N = 22%; NH4+-N = 70%). Notably, the increase in NO3−-N occurred at a faster rate compared to NH4+-N despite the latter having a higher concentration. Figure 2b shows the fractional contributions of various ON sources as a function of ON mass loading; these results are discussed in Section “Source apportionment by PMF analysis”.
a Fractional contributions of NH4+-N, NO3−-N, and ON to bulk aerosol TN as a function of TN loadings; b Fractional contributions of the 14 ON source factors to bulk aerosol ON as a function of ON loadings. The probability density of aerosol TN or ON mass concentration is shown as the white line, which sums to 100% in each subpanel. Datapoints during the Chinese New Year (CNY) holiday period are excluded.
Differential dependence of aerosol IN and ON on RH, temperature, and NOx
Figure 3 examines the dependence of aerosol IN and ON on RH, temperature, and NOx through showing the variations of ON/TN, NO3−-N, NH4+-N, and ON with respect to these three parameters. Notably, a general anti-correlation was observed between aerosol IN species (NO3−-N and NH4+-N) and RH when RH > 50%. In contrast, aerosol ON concentration displayed less sensitivity to RH changes, particularly under high RH conditions (RH > 60%). The above trends of IN and ON with RH collectively resulted in a clear upward trend of the ON/TN ratio as RH increased (Fig. 3a). Note that only a very small fraction of the nighttime data points fell within the RH bin of <50% (4.8%), hence we refrain from speculating about the reasons for the notably lower NH4+ and ON concentrations observed under the dry nighttime conditions.
ON/TN ratio and mass concentrations of aerosol NO3−-N, NH4+-N, and ON as a function of ambient RH (a-1 to a-4), temperature (b-1 to b-4), and NOx mixing ratio (c-1 to c-4) during nighttime and daytime. Bins are divided considering the distribution frequency of datapoints. Square markers and horizontal lines in the boxes are the mean and median values, respectively. Boxes represent the 25th and 75th percentiles, and whiskers represent 10th and 90th percentiles.
During the daytime, aerosol IN species showed strong negative correlations with temperature, mainly due to gas-particle partitioning strongly dependent on temperature. In contrast, aerosol ON generally increased in concentration as temperature rose, during both daytime and nighttime (Figs. 3b and 4). The daytime positive correlation between ON and temperature could be attributed to the enhanced emission of precursors at higher temperatures. This is supported by the consistent trend between the α-pinene and isoprene SOA formation related ON factors and temperature (Fig. S3). Additionally, increased solar irradiation during the daytime promotes photooxidation reactions, leading to enhanced ON formation. During the nighttime, the NO3 radical can react with NO2 to form N2O5, and this reaction is strongly temperature dependent.
The equilibrium constant of the above reaction is: \({K}_{{eq}}=5.8\times {10}^{-27}\times {e}^{\frac{10,840}{T}}\) cm3/molecule49. An increase in ambient temperature favors the equilibrium of this reaction, shifting to the left, resulting in a higher concentration of NO3 radical. To be more specific, within the relevant temperature range of our observation period (5–15 °C), a temperature increase of 10 °C would lead to 3.9 times enhancement in the equilibrium coefficient of 1/Keq (=[NO3][NO2]/[N2O5]), favoring the left side of the equation. Consequently, ON production via the NO3 radical oxidation pathway would be promoted, resulting in the observed clear increase in nighttime ON concentration with temperature during our measurement.
During nighttime, elevated NOx levels notably facilitated the formation of both NO3−-N and ON. Such a strong positive correlation persisted across all the NOx ranges (Fig. 3c). In the daytime, however, the simultaneous enhancement of NO3−-N and ON with rising NOx concentrations was observed only when NOx levels were lower than 15 ppb. As NOx further increased, NO3−-N started to show a declining trend. In contrast to the nocturnal processes, daytime ON generally exhibited lower sensitivity to changes in NOx levels. These findings suggest the potential importance of the nighttime NO3 radical-initiated oxidation pathway in the formation of secondary ON. Figure S4 demonstrates a clear positive correlation between ON and the product of NOx and O3 (i.e., [NOx][O3]) exclusively during nighttime. Similarly, Xu et al.22 reported a pronounced positive correlation between WSON levels and [NOx][O3] at a forest site, further supporting the relative significance of NOx-related reactions during the night.
Source apportionment by PMF analysis
Taking advantage of our online measurements of molecular and elemental source markers, we conducted source apportionment analysis using PMF to investigate the sources and formation pathways for aerosol ON. Among the TAG-measured organic molecular markers, XRF-measured trace elements and MARGA-measured water-soluble ions, those used as PMF input data are summarized in Tables S4 and S5, respectively. Organic molecular tracers representing common sources were grouped together to reduce the number of input parameters and to avoid collinearity50. The 14-factor solution of the PMF analysis was identified as the optimal solution, considering the reasonability of source profiles, the diurnal variation patterns of each source factor, and the solution stability. Details of the PMF modeling diagnostics, including the species error fraction, source profile uncertainty, BS and DISP results are provided in Text S1. Figure 4 presents the source profiles of the resolved PM2.5 factors, including eight primary sources: (1) soil dust, (2) ship emissions, (3) industrial and coal combustion emissions, (4) cooking, (5) fireworks, (6) biomass burning, (7) sea salt, and (8) primary biological particles. Additionally, six secondary factors were resolved, including sulfate-rich, nitrate-rich, and four SOA factors, each characterized by SOA products derived from distinct precursors. The SOA-1 factor is dominated by high loadings of α-pinene SOA products and C₅–C₉ dicarboxylic acids (DCAs). The SOA-2 factor is primarily associated with SOA products from isoprene and toluene (i.e., DHOPA). The SOA-3 factor exhibits high loadings of β-caryophyllene, naphthalene, and toluene SOA products. The SOA-4 factor is distinguished by a high loading of 4-nitrocatechol, a well-known secondary formation product of catechol, which is predominantly emitted from biomass burning. Note that both SOA-2 and SOA-3 factors comprise SOA products derived from mixed biogenic and anthropogenic precursors. Their co-occurrence among the PMF-resolved factors likely reflects the influence of the common oxidants involved during their formation.
The identification of source factors was largely based on the suite of source markers used in the PMF input matrix. The soil dust factor (F1) had high abundances of crustal elements such as Ca and Fe. The profile of ship emission (F2) was characterized by high loadings of V and Ni. The third factor showed significant contributions from various metals, including Cu, Zn, As, Se, Pb, Mn, and Fe. Among these, Mn, Zn, and Pb are often associated with industrial emissions51,52, while As and Se are effective indicators for coal combustion emissions53,54. As shown in Fig. S5, most of the coal combustion tracers exhibited relatively strong correlations with the industrial emission tracers (Rp mostly >0.5, with some exceeding 0.7). This suggests that the two sources have been well-mixed due to the long-range regional transport before reaching the studied receptor site, leading to the combined source factor identified in the PMF analysis. The cooking factor (F4) contained characteristic saturated fatty acids (palmitic acid and stearic acid). The firework source (F5) exhibited high loadings of K, Cu, and Bi. This factor showed extremely high concentrations during the Chinese New Year (CNY) festival period. Biomass burning emission (F6) was identified by the highly specific organic markers, levoglucosan and mannosan. The seventh factor (F7) was characterized by high loadings of sodium (Na+) and chloride (Cl-) ions, indicating a sea salt emission source. The primary biological particles factor (F8) was represented by high loadings of glucose and mannitol, which are widely recognized as markers of primary biological emissions55. The ninth factor (F9) contained high abundant sulfate and ammonium, representing sources associated with secondary sulfate formation processes. The formation of sulfate through aqueous reactions56 suggests that secondary OAN linked with sulfate formation may indicate the aqueous phase formation of OAN, such as imidazole-like compounds. These can be produced through reactions between carbonyls and ammonia/amines/amino acids in the aqueous phase57,58,59,60. In addition, acid-base neutralization of ammonia and organic acids in deliquesced aerosols may form organic ammonium salt61. The tenth factor (F10) contains a high abundance of nitrate and ammonium, which identifies this factor to be associated with nitrate-rich process. Nitrate formation processes may produce oxidized OAN, such as organic nitrates and nitroaromatics, as they share common precursors of NOx with nitrate. Organic nitrates can be generated through hydroxyl radicals (OH)-initiated oxidation of hydrocarbons in the presence of NOx or oxidation of alkenes by nitrate radicals (NO3) during the nighttime62. Nitroaromatics can be formed through the nitration of phenols and catechols in gas and condensed phases63. Factors 11, 12, and 13 correspond to three separate SOA factors, specifically related with α-pinene SOA, isoprene SOA and DHOPA, and β-caryophyllene SOA, respectively. Factor 14 exhibited high loadings of 4-nitrocatechol (4-NC), indicating aged biomass burning emissions64.
Figure 5 shows the ON mass concentrations attributable to the individual source factors and their percentage contributions to bulk ON. We elaborate below the major primary and secondary ON sources, respectively.
a Box plots showing the distributions of ON mass concentrations for the 14 source factors. Square markers and horizontal lines in the boxes indicate the mean and median values, respectively; boxes represent the 25th and 75th percentiles, and whiskers represent 10th and 90th percentiles. b Percentage contributions of individual source factors to ON in PM2.5. c ON mass concentrations and corresponding fractional contributions of the 14 source factors to the total aerosol ON.
At the campaign-average level, soil dust was the largest primary ON source, contributing to an average of 11.7% of ON during the campaign. This finding aligns with several previous studies that have also identified dust as a significant contributor to ON, WSON or WSIN aerosols. For example, soil dust contributed to an annual average of 9.49% of total ON mass in suburban Nansha site1. At an urban roadside site in the PRD region, soil dust was estimated to comprise 28.6% of WSON24. In Xi’an, China, high levels of WSON and WSON/WSTN ratio were found during dust storm events, attributed to the input of biota-derived OA from Gobi desert65. In the eastern Mediterranean area, WSON exhibited a relatively strong association with calcite (CaCO3) in atmospheric dust29. In Sapporo, northern Japan, biological particles contained in soil dust were thought to be important WION sources37.
Biomass burning was the second dominant source among the primary ON factors, accounting for 11.3% of the total mass on average. Biomass burning as a significant source of ON is consistent with previous field observation studies. Studies based on mass spectrometric measurements reported substantial amounts of OAN molecules from biomass burning emissions13,66,67. For example, Laskin et al.13 reported that 10–20% of identified peaks in the electrospray ionization mass spectrometry (ESI/MS) spectra of solvent extracts of aerosol samples from biomass fuel test burns corresponded to nitrogen-containing organic formulas. Among them, N-heterocyclic alkaloid compounds comprised a substantial fraction. Ho et al.66 presented evidence that biomass burning acted as an important contributing source to the enrichment of free amino acids at an inland site in China. Sun et al.67 conducted an AMS-based elemental analysis of submicron aerosols in urban Beijing and found that biomass burning OA had a higher N/C ratio (0.042) compared to other primary OA factors from traffic (0.022), cooking (0.004), and coal combustion (0.008). This difference was likely due to the greater contributions of nitrogen-containing organic compounds from biomass burning. Additionally, several source analysis studies reported biomass burning as a significant source of ambient OAN. For example, Yu et al.24 conducted source apportionment of WSON using filter-based measurements and found that biomass burning accounted for ~23–51% atmospheric WSON in different environments within the PRD region in China, with higher percentage contributions observed in rural areas than urban areas. Based on AMS measurements, Xu et al.21 estimated that biomass burning contributed to 44% of bulk ON in urban Beijing during winter. Furthermore, our study observed higher concentrations of biomass burning-derived ON at night (Fig. S6f), consistent with the previous observations of enhanced presence of nighttime biomass burning in urban Shanghai, China68.
Sea salt emission was found as the third dominant source of aerosol ON, contributing 9.2% to the ON budget. ON species of biogenic origins, such as amino acids, urea, and protein, may be carried by sea salt and transported from the sea surface to the coastal air. Previous studies have also reported significant contributions of sea salt emissions to WSON28,69. It is worth noting that sea salt can also serve as a medium for absorbing semi-volatile organic species70, some of which might contain nitrogen molecules and contribute to the observed ON mass. Primary biological particle emission was also a significant source of aerosol ON, accounting for 7.8% of total ON on average. Comparable contributions of primary biological particles (10%) to ON were observed in suburban Nansha site during the winter period, while higher than that were observed in urban Tsuen Wan site (5.0%). Higher contributions of primary biological particle contributions to ON were found in PM10, which is expected as a large fraction of primary biological particles is in coarse mode rather than in fine mode71.
Our measurement period encompassed both the Chinese New Year (CNY) holiday period (11–18 February 2021) and the non-CNY period. Comparing the non-CNY and the CNY periods, we observed a significant decrease in IN concentration by 39.9% (from 1.83 to 1.10 μgN/m3) during the CNY period, while the ON concentration remained relatively stable (0.26 vs. 0.25 μgN/m3). The firework factor accounted for a campaign average of 3.5% of ON. However, during the CNY period, its percentage contribution rose to 21.0% and mass contribution to 0.055 μgN/m3. This suggests that although firework emissions are sporadic in nature, they could emerge as significant ON contributors when fireworks are active.
Other primary sources of ON were ship emissions (6.2%), industrial & coal combustion (3.1%), and cooking (1.0%). These primary sources were also identified as contributors to ON in previous field and laboratory studies deploying AMS as the measurement instrument21,67,72,73. For example, in urban Beijing during wintertime21, AMS measurements indicated that cooking and coal combustion emissions contributed to ON in the range of 5–11% and 12%, respectively.
The top three secondary ON factors were sulfate-rich factor (22.0%), α-pinene-related SOA factor (SOA-1, 11.7%), and SOA-3 (β-caryophyllene & anthropogenic SOA, 6.4%). Previous laboratory experiments and ambient observations have shown the importance of α-pinene as a biogenic precursor for the formation of organic nitrates through both OH and NO3 reaction pathways74,75. The importance of sulfate formation-related processes in contributing to ON was documented in a study conducted in a forest site in Japan22. The authors found that the anthropogenic sulfate-rich factor accounted for major fractions of WSON, representing 48 and 61% during daytime and nighttime, respectively. It was suggested that aerosol liquid water driven by anthropogenic sulfate could effectively promote WSON formation through aqueous-phase reactions involving biogenic VOCs and NH3/NH4+. Nitrate-rich factor accounted for 4.9% of total ON on average during the study period, which is comparable to those observed in urban Hong Kong (5.94%)1, urban Shanghai (4.8%)48, and suburban Guangzhou (4.28%)1, but lower than that in urban Beijing (14.3%)1. The discrepancy in nitrate-rich contributions could be attributed to the higher NOx levels. The other two SOA factors (i.e., isoprene-SOA factor and nitrocatechol formation factor) made minor contributions (0.5% and 0.4%, respectively). Overall, these six secondary formation factors constituted 46% of bulk ON.
Figure 2b presents the fractional contributions of different ON sources as a function of the total ON mass loading. The fractional contributions of primary ON factors tended to be lower when the ON mass loading was higher. In contrast, the secondary ON factors increased their contributions from 35 to 63% as the ON loading increased from ~0.1 to ~0.5 µgN/m3. Notably, there was a significant enhancement in contribution from the sulfate-rich and α-Pinene-SOA factor during hours of higher ON loadings (~0.3 and ~0.2 µgN/m3 and higher). These findings suggest that secondary formation processes, particularly those related to biogenic SOA formation, played a key role in ON pollution episodes at this suburban site.
To estimate the abundance of aerosol ON and atmospheric ON deposition, modeling studies commonly rely on OC emission inventories supplemented with empirically assumed ON/OC ratios for each primary emission source to derive ON from primary emissions76,77. However, due to the scarcity of direct measurement data, these assumed ON/OC ratios are associated with large uncertainties. Therefore, it is valuable to examine the ON/OC ratios in the PMF-resolved source factors, as these source-specific ratios can serve as reference values to improve the representation of ON in modeling studies.
Table 1 summarizes contributions to ON and the ON-to-OC ratios of major source factors that contributed more than 5% of total ON. The ON/OC ratio for each source factor was calculated by dividing the ON concentration by the OC concentration in each source factor. Notably, the ON/OC ratio in the dust (0.085), and SOA-3 (0.0848) were the highest among all the ON factors, consistent with reported values ranging from,0.097 to 0.136 in southern and northern China1. The sulfate-rich factor showed the next highest ON/OC ratio (0.0814), followed by sea salt (0.0771), α-pinene-related SOA formation (0.0565), primary biological particles (0.0565), ship emissions (0.0557) and biomass burning (0.0472). A comparison of ON/OC ratio for individual source factors with those from other studies is shown in Fig. S8. The ON/OC ratio in the biomass burning factor in the current study is similar to that of biomass burning OA (BBOA) as determined by AMS measurements in several studies (e.g., 0.049–0.096 during Beijing winter)21, but lower than those in the rural areas in PRD during autumn (0.07)78. Note that AMS studies utilize the “Improved-Ambient” method to determine the atomic ON/OC ratio through elemental analysis79. We calculated the OA factor’s corresponding ON/OC ratio by multiplying the atomic N/C ratio by the ratio of the molar masses of nitrogen and carbon (14/12). The ON/OC ratio of the cooking emissions factor in our work (0.053) is close to the results in Hong Kong (0.052)1, but is much higher than the ratio measured by AMS for laboratory-generated cooking emissions (0.006–0.039)73, and those from ambient studies (0.005–0.018)21,67.
Case studies of episodic aerosol ON events
To gain a better understanding of the key atmospheric processes and influential factors affecting OAN, we selected five occasions (referred to as episodes EP1–EP5) with ON exceeding the campaign average by 25% or more for in-depth case studies. The selected periods encompassed events characterized by elevated ON alone (i.e., EP4, and EP5) or significant enhancement of IN and ON in sync (i.e., EP1, EP2, and EP3). Detailed observation data and ON source apportionment results can be found in Tables 2, S8 and S9. Figure 6 presents the time series of nitrogenous aerosols, along with meteorological parameters, gases, three PMF-resolved major ON factors, and their characteristics tracers during these five episodic cases.
Cases EP1 to EP3 were characterized by simultaneous enhancement of both IN and ON. All three episodes occurred when the dominant wind shifted to the northern direction and increased wind speed (4.0–5.8 m/s). TN concentrations increased to ~2 times the campaign-average value during these episodes. The levels of nitrogenous aerosols generally rose with the total PM2.5 mass, indicating the influence of regional air pollution transport from continental areas to the north. However, the leading sources of ON were different among these cases.
EP1 and EP3 exemplified typical cases of secondary pollution associated with regional transport. The two episodes persisted for 60 and 40 h, respectively. Despite the NOx level remaining around the average value, both IN and ON showed prominent concentration elevation. The results of source apportionment revealed that sulfate-rich factor emerged as the major contributor to ON, accounting for 32.4% and 26.6% of ON during EP1 and EP3, respectively (Table S8). Pollutants were brought by the wind from the northern continental areas of the PRD region, undergoing secondary formation during transport. EP3 showed distinct daytime peaks of IN and ON, while exhibiting relatively strong correlations between nitrogenous aerosols and O3 (Rp = 0.57–0.60). This appeared to suggest that daytime photochemical processes played a role.
EP2 served as a clear example of nighttime ON formation, possibly due to the conversion of NOx precursor, as evidenced by the strong correlation between ON and NOx (Rp = 0.94). During EP2, NOx levels reached 25.6 ppb, three times the measurement average. Additionally, the elevated ON concentration was primarily associated with α-pinene SOA formation processes (Fig. 6), which contributed 21.0% of the ON source during this episode of EP2. This suggests the nighttime ON episode was likely triggered by the formation of organic nitrates through the reactions between α-pinene and the NO3 radical. Concentrations of the top two most abundant NACs, 4-NC and 4-nitrophenol (4-NP), also showed significant enhancements during the nighttime episode (EP2), consistent with the previously proposed mechanism that NACs can be formed during nighttime through reactions initiated by NO3 radicals in the presence of NOx80,81.
Both EP4 and EP5 exhibited prominent ON enhancement, with ON fraction in TN of ~16%, notably higher than the average ON/TN ratio of 12.9% during the whole measurement period. EP4 showed extremely high loadings of α-pinene SOA tracers (43.4 ng/m3, 4.4 times of the average concentration). This ON episode was primarily driven by α-pinene SOA formation processes (28.6%). In EP5, an abrupt increase of biomass burning tracers, levoglucosan, and mannosan was observed, coinciding with a short-term shift in wind direction from the eastern marine direction to the northern inland direction. Dramatic increases were also observed in 4-NC and 4-NP concentrations, with a strong to moderate correlation with levoglucosan (Rp = 0.84 and 0.68, respectively). During EP5, biomass burning became the dominant contributor to ON, accounting for 0.12 µgN/m3 or 28.0% of ON. The abundance of other major SOA tracers remained low during this period (Fig. 6).
The different variations in aerosol IN and ON during EP4 and EP5 provided an illustrative example of the decoupling of sources or formation processes of IN and ON despite their sharing some common precursors. Aerosol ON originates from a complex interplay of primary and secondary sources, leading to large variations in its source composition. The online bihourly measurement of nitrogenous aerosols enabled the detection of rapid changes in ON sources and formation pathways, which, in turn, will help develop effective ON pollution control policies in the future.
Discussion
Previous understanding of aerosol ON sources has mainly derived from WSON measurements due to the limited measurement of WION. Our newly acquired ability to quantify the total aerosol ON, including both WSON and WION, enables a more complete assessment of ON sources and their environmental and health impacts. By integrating bihourly measurements of molecular markers and elemental tracers, this study achieves quantitative source apportionment of ON and reveals its diurnal and episodic dynamics. Our results show that, at this suburban site in winter, primary emissions and secondary formation processes contributed comparably to aerosol ON, with major sources including sulfate formation processes (22.0%), α-pinene-derived SOA (11.7%), soil dust (11.7%), biomass burning (11.3%), sea salt (9.21%), and primary biological particles (7.80%). Episodes of elevated ON were often dominated by one or two of these sources. Notably, ON and IN levels did not always rise simultaneously, underscoring their complex relationship despite shared precursors. These findings provide new constraints for understanding ON formation mechanisms and their environmental relevance.
Field-based source apportionment of ON has significant implications for refining ON emission inventories and atmospheric models. Current gaps in our knowledge of ON emission inventories and atmospheric transformations greatly hinder the improvement of modeling accuracy for aerosol ON. Given that the relative importance of primary and secondary ON sources is likely to vary across urban, suburban, rural, forest, and marine environments, field measurements in different locations of distinct environmental characteristics are essential for validating laboratory-based mechanisms and evaluating regional and global OAN models. Future research should combine bulk ON quantification for mass closure with molecular-level analysis for detailed composition and source attribution.
Finally, the detailed source and temporal data presented here are valuable for projecting how OAN abundance and composition may evolve under climate change. As climate-driven shifts in temperature, humidity, and extreme events affect ON emissions and chemical transformations, such real-world observations are essential for predicting future air quality and informing effective management strategies.
Method
Sampling site description
The ambient measurement was conducted at the Air Quality Research Supersite on the campus of the Hong Kong University of Science and Technology (HKUST, 22.34°N 114.27°E), a suburban site on the east coast of Hong Kong in the Pearl River Delta (PRD) in China (Fig. S9). The site is a regional pollution receptor site, with little local emissions in the surrounding area82,83. Minor local sources include a dormitory construction site and a small canteen nearby, and the Clear Water Bay Road outside the campus at ~500 m distance. The sampling period was from 29 November 2020 to 28 February 2021.
Aerosol organic nitrogen quantification
The online aerosol IN & ON analyzer system integrates a real-time aerosol carbon analyzer based on thermal evolution method (RT-3131, Sunset Laboratory, USA) and a chemiluminescent NOx analyzer (Model 42i, Thermo Scientific, USA). A schematic component diagram is shown in Fig. S10. A 6-step temperature program (i.e., 150, 180, 300, 400, 500, and 800 °C) was applied to thermally release various aerosol components in a carrier gas of 10% O2/He. Inorganic and organic nitrogenous components have distinct thermal evolution behaviors. Multivariate curve resolution analysis was then conducted to resolve the nitrogen signal into IN and ON components based on their thermal evolution characteristics. Details of the aerosol IN and ON quantification are described in Li et al.47 and Yu et al.35 The analyzer has a bi-hourly time resolution, with sampling lasting 1 h, followed by an hour of analysis. Routine calibrations for the online aerosol nitrogen analyzer system were conducted with (NH4)2SO4 standard each month47. The sampling started at even hours (e.g., 00:00, 02:00). Throughout the measurement period, a total of 836 pairs of IN and ON data points were collected.
Source-specific tracer measurements and other collocated instruments
An array of instruments was deployed. Meteorological parameters, including temperature, relative humidity (RH), wind speed (WS), and wind direction (WD) were monitored by an AWS tower (Model 6000, Belfort Instrument Company, USA). Organic carbon (OC) and elemental carbon (EC) were measured by a semi-continuous OC-EC field analyzer (RT-3179, Sunset Laboratory, USA). Trace elements were quantified with an online XRF spectrometer (Xact® 625i, Cooper Environmental Services, USA). Water-soluble ions were determined by the Monitor for AeRosols and GAses in ambient Air (MARGA, Metrohm Applikon, The Netherlands). Organic molecular markers in PM2.5 were measured using the thermal desorption aerosol gas chromatography–mass spectrometry system (TAG, Aerodyne Research Inc., USA). Detailed information about the TAG system, including its components and working principle, can be found in previous studies84,85,86. During the campaign, the TAG system collected hourly samples at even hours, synchronized with the online IN & ON analyzer system. Details of all the instruments are provided in Table S10.
Source apportionment analysis using PMF
PMF is a widely used receptor model for identifying sources and quantifying their contributions to ambient air pollutants. Based on mass conservation and chemical mass balance between emission sources and receptor sites87, the PMF model decomposes the concentration matrix of ambient pollutants into the source contribution matrix, the factor profile matrix, and the residual matrix. In this study, the USEPA-PMF version 5.0 tool was used to carry out the source apportionment analysis of PM2.5 and ON. The input parameters include ON, OC, EC, water soluble ions, trace elements, and organic molecular tracers in PM2.5. The robustness of the PMF solution is examined through two uncertainty estimation methods: bootstrapping (BS) and displacement (DISP). BS checks uncertainties related with random errors by mapping the resampled data matrix to the base factors to provide the reproducibility of different factors. DISP explores the rotational ambiguity of the PMF solution through displacing each element in the factor profile matrix.
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the corresponding authors (jian.yu@ust.hk).
References
Yu, X. et al. New measurements reveal a large contribution of nitrogenous molecules to ambient organic aerosol. Npj Clim. Atmos. Sci. 7, 72 (2024).
Jickells, T., Baker, A. R., Cape, J. N., Cornell, S. E. & Nemitz, E. The cycling of organic nitrogen through the atmosphere. Philos. Trans. R. Soc. B Biol. Sci. 368, 20130115 (2013).
Elser, J. J. et al. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 10, 1135–1142 (2007).
Gruber, N. & Galloway, J. N. An Earth-system perspective of the global nitrogen cycle. Nature 451, 293–296 (2008).
Mohr, C. et al. Contribution of nitrated phenols to wood burning brown carbon light absorption in Detling, United Kingdom during winter time. Environ. Sci. Technol. 47, 6316–6324 (2013).
Xie, M. et al. Light absorption of secondary organic aerosol: composition and contribution of nitroaromatic compounds. Environ. Sci. Technol. 51, 11607–11616 (2017).
Yang, Z. M., Tsona, N. T., George, C. & Du, L. Nitrogen-containing compounds enhance light absorption of aromatic-derived brown carbon. Environ. Sci. Technol. 56, 4005–4016 (2022).
Yao, L. et al. Atmospheric new particle formation from sulfuric acid and amines in a Chinese megacity. Science 361, 278–281 (2018).
Lee, B. H. et al. Highly functionalized organic nitrates in the southeast United States: contribution to secondary organic aerosol and reactive nitrogen budgets. Proc. Natl. Acad. Sci. USA 113, 1516–1521 (2016).
Landvik, N. E. et al. Effects of nitrated-polycyclic aromatic hydrocarbons and diesel exhaust particle extracts on cell signalling related to apoptosis: possible implications for their mutagenic and carcinogenic effects. Toxicology 231, 159–174 (2007).
Kovacic, P. & Somanathan, R. Nitroaromatic compounds: environmental toxicity, carcinogenicity, mutagenicity, therapy and mechanism. J. Appl. Toxicol. 34, 810–824 (2014).
Cornell, S. E., Jickells, T. D., Cape, J. N., Rowland, A. P. & Duce, R. A. Organic nitrogen deposition on land and coastal environments: a review of methods and data. Atmos. Environ. 37, 2173–2191 (2003).
Laskin, A., Smith, J. S. & Laskin, J. Molecular characterization of nitrogen-containing organic compounds in biomass burning aerosols using high-resolution mass spectrometry. Environ. Sci. Technol. 43, 3764–3771 (2009).
Matsumoto, K., Yamamoto, Y., Kobayashi, H., Kaneyasu, N. & Nakano, T. Water-soluble organic nitrogen in the ambient aerosols and its contribution to the dry deposition of fixed nitrogen species in Japan. Atmos. Environ. 95, 334–343 (2014).
Neff, J. C., Holland, E. A., Dentener, F. J., McDowell, W. H. & Russell, K. M. The origin, composition and rates of organic nitrogen deposition: a missing piece of the nitrogen cycle? Biogeochemistry 57, 99–136 (2002).
Wang, Y. et al. Enhancement in particulate organic nitrogen and light absorption of humic-like substances over Tibetan Plateau due to long-range transported biomass burning emissions. Environ. Sci. Technol. 53, 14222–14232 (2019).
Zhang, X., Xu, J., Kang, S., Liu, Y. & Zhang, Q. Chemical characterization of long-range transport biomass burning emissions to the Himalayas: insights from high-resolution aerosol mass spectrometry. Atmos. Chem. Phys. 18, 4617–4638 (2018).
Bates, K. H., Burke, G. J. P., Cope, J. D. & Nguyen, T. B. Secondary organic aerosol and organic nitrogen yields from the nitrate radical (NO3) oxidation of alpha-pinene from various RO2 fates. Atmos. Chem. Phys. 22, 1467–1482 (2022).
Cape, J. N., Cornell, S. E., Jickells, T. D. & Nemitz, E. Organic nitrogen in the atmosphere—where does it come from? A review of sources and methods. Atmos. Res. 102, 30–48 (2011).
Facchini, M. C. et al. Important source of marine secondary organic aerosol from biogenic amines. Environ. Sci. Technol. 42, 9116–9121 (2008).
Xu, W. et al. Seasonal characterization of organic nitrogen in atmospheric aerosols using high resolution aerosol mass spectrometry in Beijing, China. ACS Earth Sp. Chem. 1, 673–682 (2017).
Xu, Y. et al. Aerosol liquid water promotes the formation of water-soluble organic nitrogen in submicrometer aerosols in a suburban forest. Environ. Sci. Technol. 54, 1406–1414 (2020).
Tsagkaraki, M. et al. Spatiotemporal variability and sources of aerosol water-soluble organic nitrogen (WSON), in the Eastern Mediterranean. Atmos. Environ. 246, 118144 (2021).
Yu, X. et al. Water soluble organic nitrogen (WSON) in ambient fine particles over a megacity in South China: spatiotemporal variations and source apportionment. J. Geophys. Res. Atmos. 122, 13045–13060 (2017).
Vodička, P., Kawamura, K., Schwarz, J., Kunwar, B. & Ždímal, V. Seasonal study of stable carbon and nitrogen isotopic composition in fine aerosols at a Central European rural background station. Atmos. Chem. Phys. 19, 3463–3479 (2019).
Mace, K. A., Artaxo, P. & Duce, R. A. Water-soluble organic nitrogen in Amazon Basin aerosols during the dry (biomass burning) and wet seasons. J. Geophys. Res. Atmos. 108, D16 (2003).
Miyazaki, Y., Fu, P., Ono, K., Tachibana, E. & Kawamura, K. Seasonal cycles of water-soluble organic nitrogen aerosols in a deciduous broadleaf forest in northern Japan. J. Geophys. Res. Atmos. 119, 1440–1454 (2014).
Chen, H. Y. et al. Size fractionation and molecular composition of water-soluble inorganic and organic nitrogen in aerosols of a coastal environment. J. Geophys. Res. Atmos. 115, D22307 (2010).
Mace, K. A., Kubilay, N. & Duce, R. A. Organic nitrogen in rain and aerosol in the eastern Mediterranean atmosphere: an association with atmospheric dust. J. Geophys. Res. Atmos. 108, D10 (2003).
Luo, L. et al. Sources of reactive nitrogen in marine aerosol over the Northwest Pacific Ocean in spring. Atmos. Chem. Phys. 18, 6207–6222 (2018).
Miyazaki, Y., Kawamura, K., Jung, J., Furutani, H. & Uematsu, M. Latitudinal distributions of organic nitrogen and organic carbon in marine aerosols over the western North Pacific. Atmos. Chem. Phys. 11, 3037–3049 (2011).
Nakamura, T., Ogawa, H., Maripi, D. & Uematsu, M. Contribution of water soluble organic nitrogen to total nitrogen in marine aerosols over the East China Sea and western North Pacific. Atmos. Environ. 40, 7259–7264 (2006).
Violaki, K. & Mihalopoulos, N. Water-soluble organic nitrogen (WSON) in size-segregated atmospheric particles over the Eastern Mediterranean. Atmos. Environ. 44, 4339–4345 (2010).
Bhattarai, H. et al. Nitrogen speciation and isotopic composition of aerosols collected at himalayan forest (3326 m a.s.l.): seasonality, sources, and implications. Environ. Sci. Technol. 53, 12247–12256 (2019).
Yu, X. et al. Simultaneous determination of aerosol inorganic and organic nitrogen by thermal evolution and chemiluminescence detection. Environ. Sci. Technol. 55, 11579–11589 (2021).
Matsumoto, K., Sakata, K. & Watanabe, Y. Water-soluble and water-insoluble organic nitrogen in the dry and wet deposition. Atmos. Environ. 218, 117022 (2019).
Pavuluri, C. M., Kawamura, K. & Fu, P. Q. Atmospheric chemistry of nitrogenous aerosols in northeastern Asia: biological sources and secondary formation. Atmos. Chem. Phys. 15, 9883–9896 (2015).
Li, Q. & Yu, J. Z. Determination of total aerosol nitrogen by thermal evolution. Aerosol Sci. Technol. 38, 382–390 (2004).
Wang, Q. Q. et al. Source apportionment of PM2.5 using hourly measurements of elemental tracers and major constituents in an urban environment: investigation of time-resolution influence. J. Geophys. Res. Atmos. 123, 5284–5300 (2018).
Rastogi, N., Zhang, X., Edgerton, E. S., Ingall, E. & Weber, R. J. Filterable water-soluble organic nitrogen in fine particles over the southeastern USA during summer. Atmos. Environ. 45, 6040–6047 (2011).
Wu, L. Source forensics of inorganic and organic nitrogen using δ15N for tropospheric aerosols over Mt. Tai.Npj Clim. Atmos. Sci. 4, 8 (2021).
Ng, N. L. et al. Nitrate radicals and biogenic volatile organic compounds: oxidation, mechanisms, and organic aerosol. Atmos. Chem. Phys. 17, 2103–2162 (2017).
Reyes-Villegas, E. et al. Simultaneous aerosol mass spectrometry and chemical ionisation mass spectrometry measurements during a biomass burning event in the UK: insights into nitrate chemistry. Atmos. Chem. Phys. 18, 4093–4111 (2018).
Salvador, C. M. G. et al. Ambient nitro-aromatic compounds—biomass burning versus secondary formation in rural China. Atmos. Chem. Phys. 21, 1389–1406 (2021).
Xu, W. et al. Estimation of particulate organic nitrates from thermodenuder–aerosol mass spectrometer measurements in the North China Plain. Atmos. Meas. Tech. 14, 3693–3705 (2021).
Yu, X., Wong, Y. K. & Yu, J. Z. Abundance and sources of organic nitrogen in fine (PM2.5) and coarse (PM2.5-10) particulate matter in urban Hong Kong. Sci. Total Environ. 901, 165880 (2023).
Li, J. et al. Online measurement of aerosol inorganic and organic nitrogen based on thermal evolution and chemiluminescent detection. Atmos. Environ. 271, 118905 (2022).
Yu, X. et al. First online observation of aerosol total organic nitrogen at an urban site: insights into the emission sources and formation pathways of nitrogenous organic aerosols. J. Geophys. Res. Atmos. 128, e2023JD038921 (2023).
Burkholder, J. B. S. et al. Chemical Kinetics and Photochemical Data for Use in Atmospheric Studies; Evaluation Number 19 (Jet Propulsion Laboratory, National Aeronautics and Space Administration, 2020).
Wang, Q. et al. Impact of secondary organic aerosol tracers on tracer-based source apportionment of organic carbon and PM2.5: a case study in the Pearl River Delta, China. ACS Earth Sp. Chem. 1, 562–571 (2017).
Dall’Osto, M. et al. Hourly elemental concentrations in PM2.5 aerosols sampled simultaneously at urban background and road site during SAPUSS – diurnal variations and PMF receptor modelling. Atmos. Chem. Phys. 13, 4375–4392 (2013).
Moffet, R. C. et al. Characterization of aerosols containing Zn, Pb, and Cl from an industrial region of Mexico City. Environ. Sci. Technol. 42, 7091–7097 (2008).
Chen, J. et al. Atmospheric emissions of F, As, Se, Hg, and Sb from coal-fired power and heat generation in China. Chemosphere 90, 1925–1932 (2013).
Tian, H. Z. et al. Trend and characteristics of atmospheric emissions of Hg, As, and Se from coal combustion in China, 1980–2007. Atmos. Chem. Phys. 10, 11905–11919 (2010).
Gosselin, M. I. et al. Fluorescent bioaerosol particle, molecular tracer, and fungal spore concentrations during dry and rainy periods in a semi-arid forest. Atmos. Chem. Phys. 16, 15165–15184 (2016).
Yu, J. Z., Huang, X. F., Xu, J. H. & Hu, M. When aerosol sulfate goes up, so does oxalate: Implication for the formation mechanisms of oxalate. Environ. Sci. Technol. 39, 128–133 (2005).
Galloway, M. M. et al. Glyoxal uptake on ammonium sulphate seed aerosol: reaction products and reversibility of uptake under dark and irradiated conditions. Atmos. Chem. Phys. 9, 3331–3345 (2009).
Nozière, B., Dziedzic, P. & Córdova, A. Products and kinetics of the liquid-phase reaction of glyoxal catalyzed by ammonium ions (NH4+). J. Phys. Chem. A 113, 231–237 (2009).
Zhang, G. H. et al. High secondary formation of nitrogen-containing organics (NOCs) and its possible link to oxidized organics and ammonium. Atmos. Chem. Phys. 20, 1469–1481 (2020).
Liu, X. D. et al. Secondary formation of atmospheric brown carbon in China haze: implication for an enhancing role of ammonia. Environ. Sci. Technol. 57, 11163–11172 (2023).
Paciga, A. L., Riipinen, I. & Pandis, S. N. Effect of ammonia on the volatility of organic diacids. Environ. Sci. Technol. 48, 13769–13775 (2014).
Perring, A. E., Pusede, S. E. & Cohen, R. C. An observational perspective on the atmospheric impacts of alkyl and multifunctional nitrates on ozone and secondary organic aerosol. Chem. Rev. 113, 5848–5870 (2013).
Harrison, M. A. J. et al. Nitrated phenols in the atmosphere: a review. Atmos. Environ. 39, 231–248 (2005).
Chow, K. S., Huang, X. H. H. & Yu, J. Z. Quantification of nitroaromatic compounds in atmospheric fine particulate matter in Hong Kong over 3 years: field measurement evidence for secondary formation derived from biomass burning emissions. Environ. Chem. 13, 665–673 (2016).
Wang, G. H. et al. Impact of Gobi desert dust on aerosol chemistry of Xi’an, inland China during spring 2009: differences in composition and size distribution between the urban ground surface and the mountain atmosphere. Atmos. Chem. Phys. 13, 819–835 (2013).
Ho, K. F. et al. Characteristics of water-soluble organic nitrogen in fine particulate matter in the continental area of China. Atmos. Environ. 106, 252–261 (2015).
Sun, Y. et al. Primary and secondary aerosols in Beijing in winter: sources, variations and processes. Atmos. Chem. Phys. 16, 8309–8329 (2016).
Li, R. et al. Source apportionment of PM2.5 in Shanghai based on hourly organic molecular markers and other source tracers. Atmos. Chem. Phys. 20, 12047–12061 (2020).
Kunwar, B. & Kawamura, K. One-year observations of carbonaceous and nitrogenous components and major ions in the aerosols from subtropical Okinawa Island, an outflow region of Asian dusts. Atmos. Chem. Phys. 14, 1819–1836 (2014).
AzadiAghdam, M. et al. On the nature of sea salt aerosol at a coastal megacity: insights from Manila, Philippines in Southeast Asia. Atmos. Environ. 216, 116922 (2019).
Pöschl, U. et al. Rainforest aerosols as biogenic nuclei of clouds and precipitation in the Amazon. Science 329, 1513–1516 (2010).
Reyes-Villegas, E. et al. Online chemical characterization of food-cooking organic aerosols: implications for source apportionment. Environ. Sci. Technol. 52, 5308–5318 (2018).
Xu, W. et al. Mass spectral characterization of primary emissions and implications in source apportionment of organic aerosol. Atmos. Meas. Tech. 13, 3205–3219 (2020).
Hallquist, M. et al. The formation, properties and impact of secondary organic aerosol: current and emerging issues. Atmos. Chem. Phys. 9, 5155–5236 (2009).
Pratt, K. A. et al. Contributions of individual reactive biogenic volatile organic compounds to organic nitrates above a mixed forest. Atmos. Chem. Phys. 12, 10125–10143 (2012).
Kanakidou, M. et al. Atmospheric fluxes of organic N and P to the global ocean. Global Biogeochem. Cycles 26, GB3026 (2012).
Li, Y. M. et al. Dissecting the contributions of organic nitrogen aerosols to global atmospheric nitrogen deposition and implications for ecosystems. Natl. Sci. Rev 10, nwad244 (2023).
Huang, X. F. et al. Characterization of submicron aerosols at a rural site in Pearl River Delta of China using an aerodyne high-resolution aerosol mass spectrometer. Atmos. Chem. Phys. 11, 1865–1877 (2011).
Canagaratna, M. R. et al. Elemental ratio measurements of organic compounds using aerosol mass spectrometry: characterization, improved calibration, and implications. Atmos. Chem. Phys. 15, 253–272 (2015).
Finewax, Z., de Gouw, J. A. & Ziemann, P. J. Identification and quantification of 4-nitrocatechol formed from OH and NO3 radical-initiated reactions of catechol in air in the presence of NOx: implications for secondary organic aerosol formation from biomass burning. Environ. Sci. Technol. 52, 1981–1989 (2018).
Wang, Y. J. et al. The formation of nitro-aromatic compounds under high NOx and anthropogenic VOC conditions in urban Beijing, China. Atmos. Chem. Phys. 19, 7649–7665 (2019).
Wang, Q. et al. Chemical evolution of secondary organic aerosol tracers during high-PM2.5 episodes at a suburban site in Hong Kong over 4 months of continuous measurement. Atmos. Chem. Phys. 22, 11239–11253 (2022).
Wang, S. et al. Molecular and elemental tracers-based receptor modeling of PM2.5 in Suburban Hong Kong with hourly time-scale air quality considerations. J. Geophys. Res. Atmos. 128, e2023JD039875 (2023).
Isaacman, G. et al. Online derivatization for hourly measurements of gas- and particle-phase semi-volatile oxygenated organic compounds by thermal desorption aerosol gas chromatography (SV-TAG). Atmos. Meas. Tech. 7, 4417–4429 (2014).
Wang, Q. et al. Hourly measurements of organic molecular markers in Urban Shanghai, China: primary organic aerosol source identification and observation of cooking aerosol aging. ACS Earth Sp. Chem. 4, 1670–1685 (2020).
Williams, B. J., Goldstein, A. H., Kreisberg, N. M. & Hering, S. V. An in-situ instrument for speciated organic composition of atmospheric aerosols: thermal desorption aerosol GC/MS-FID (TAG). Aerosol Sci. Tech. 40, 627–638 (2006).
Paatero, P. & Tapper, U. Positive matrix factorization: a non-negative factor model with optimal utilization of error estimates of data values. Environmetrics 5, 111–126 (1994).
Acknowledgements
This work was supported by the Research Grants Council of Hong Kong (16304519, 16307820, 16213222 and AoE/P-601/23-N) and the Center for Ocean Research in Hong Kong and Macau, a joint research center for ocean research between Laoshan Laboratory and HKUST (QNLM20SC01-T). Special thank goes to Xin Feng, Penggang Zheng, and Prof. Zhe Wang for providing the gaseous pollutant data at the HKUST supersite.
Author information
Authors and Affiliations
Contributions
J.L. and J.Z.Y. designed the experiment. J.L., S.W., X.Y., Y.Y.C., and H.C. collected the data used in this study. J.L. and S.W. analyzed and wrote the manuscript. J.Z.Y. edited the manuscript and acquired funding. All authors reviewed and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, J., Wang, S., Yu, X. et al. Molecular and elemental markers-based source apportionment of organic nitrogen in PM2.5 at a suburban site in Hong Kong. npj Clean Air 1, 27 (2025). https://doi.org/10.1038/s44407-025-00026-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44407-025-00026-5