Abstract
Exceptional properties of graphene have triggered intensive research on other 2D materials. Surface plasmon is another subject being actively explored for many applications. Herein we report a new class of core-shell nanostructure in which the shell is made of a 2D material for effective plasmonic propagation. We have designed a much enhanced chemical sensor made of plasmonic Agx@(2D-WO3) that combines above advantages. Specifically, the sensor response increases from 38 for Agx-WO3 mixture to 217 for the Agx@(2D-WO3) core-shell structure; response and recovery time are shortened considerably to 2 and 5 seconds; and optimum sensor working temperature is lowered from 370°C to 340°C. Light irradiation is found to increase the Agx@(2D-WO3) sensor response, particularly at blue wavelength where it resonates with the absorption of Ag nanoparticles. Raman scattering shows significantly enhanced intensity for both the 2D-WO3 shell and surface adsorbates. Both the resonance sensor enhancement and the Raman suggest that the improved sensor performance is due to nanoplasmonic mechanism. It is demonstrated that (1) 2D material can be used as the shell component of a core-shell nanostructure and (2) surface plasmon can effectively boost sensor performance.
Similar content being viewed by others
Introduction
Owing to its unique one-atom thick 2D structure, graphene has shown exceptional characteristics in mechanical, thermal, electronic and optical properties1,2, demonstrating its potential to revolutionize many applications ranging from teraherz frequency manipulation3, fuel cells4, supercapacitors5, highly sensitive biosensors6,7, solar cells8 to various sensor and actuator applications9. These intriguing prospects have triggered intensive research on graphene to expand to other 2D materials2,10 such as isolated monolayer atomic crystals, MoS2, WS2, WO3, WSe2, other dichalcogenides and layered oxides, hexagonal boron nitride, etc. In fact, it is expected that the research effort will reach the same intensity as that on graphene2.
There are few limited 2D oxides known so far including mono-layers of TiO2, MoO3, WO3, mica and a few perovskite-like crystals11,12. As oxides, they are less susceptible to air but tend to partially lose oxygen at elevated temperature and chemisorb organic compounds. In addition, these monoatomic-layer oxides often have quantum effect enhanced characteristic such as lower dielectric constants, larger bandgap, charge density waves, etc.13,14, likely leading to much better chemical sensor performance. As strong chemical bonds provide in-plane stability, whereas much weaker van-der-Waals-like forces allow large spacing between layers, 2D crystals provide enough room for gas molecules to enter into the spacing between layers for improved sensor sensitivity. WO3, a wide bandgap semiconductor with its graphene-like 2D layered structure and good sensor response to a variety of volatile compounds, is a perfect model oxide in this category.
Meanwhile, metal particles with nanometer dimension have shown many unprecedented attributes, such as surface plasmon (SP), light-scattering, catalysis, biologic recognition, etc.15. Surface plasmons (SPs) are electromagnetic waves coupled to the collective oscillations of electrons in metal nanoparticles (NPs). Among well-known plasmonic metal elements, including Au, Cu, Al and Li, Ag is considered the most important one for its ability to support SPs across spectrum from 300 to 1200 nm16,17. In fact, its enhanced efficiencies have been demonstrated for solar cells, surface-enhanced Raman scattering, fluorescence, plasmonic antennas and circuitry16.
Consequently, Agx@WO3 nanostructure is of particular interest as (1) Agx nanoparticle (NP) would form an effective core to provide strong localized surface plasmon (LSP) excited across visible spectrum16; (2) LSP can be optimized by adjusting the Agx core diameter18; (3) WO3 is an excellent gas sensor material by itself; (4) Agx@WO3 is expected to be a good Schottky junction sensor; (5) the well-known layered structure of WO3 at nanometer thickness may provide quantum effect enhanced sensor performance; and (6) the combined structure of nano Agx core and the layered WO3 shell could lead to a new generation of quantum effect enhanced gas sensors.
Here we report a new class of core-shell nanostructure (CSNS) in which the shell is made of a 2D layered material for effective LSP propagation. Using the concept, we have designed and fabricated a much enhanced chemical sensor made of plasmonic Agx@(2D-WO3) CSNS – a product combined advantages of both 2D-WO3 and LSP of Ag nanoparticle (NP). The sensor response increases from 38, for the best sensor based on simple mixture of WO3 and Ag NPs, to 217 for sensor based on Agx@(2D-WO3); under a weak blue LED irradiation at only 17 mW/cm2, the Ag(25nm)@(2D-WO3) based sensor response increases further to 408; response and recovery time are shortened considerably to 2 and 5 seconds. Moreover, optimum sensor working temperature is lowered from 370°C to 340°C. It is also found that white light irradiation at ~1 sun intensity increases Agx@(2D-WO3) sensor response by more than 3 times comparing to its dark measurement. Raman scattering of the CSNS shows significantly enhanced intensity for both the 2D-WO3 shell and ethanol molecules adsorbed on it, suggesting that the enhancement is due to nanoplasmonic mechanism. It shows that 2D material can be used as the shell component of a CSNS and this may represent a new class of material. Moreover, it also appears that this is the first time for LSP being used to effectively boost chemical sensor performance. It is expected that this may pave a way for different combinations of nano metal core and 2D-semiconductor shell materials and new LSP enhanced nano-devices.
Results
Fabrication of Agx@(2D-WO3) CSNS
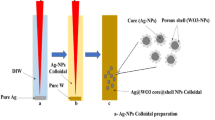
Size-controlled Ag NPs with diameter ranging from ~25 nm to ~60 nm, referenced by formula Ag(diameter) as Ag(25nm) and Ag(60nm), were synthesized using a well-developed method19. As illustrated in Supplementary Fig. S1a online, the Ag NPs were dispersed into a Na2WO4 solution first, upon adding HNO3, Agx@H2WO4 precipitate was formed. An extensive high temperature treatment process was then used to remove water from the precipitate to turn it into Agx@WO3 CSNS. For comparison, nano Agx-WO3 mixture was prepared by simply adding preformed WO3 powder into Ag NP colloid, separating, cleaning and drying using similar procedures.
X-Ray diffraction of pure WO3, Agx-WO3 mixture and Agx@(2D-WO3) samples
Figure 1 shows X-ray diffraction (XRD) patterns between 22° ~ 25° of pure WO3, Agx-WO3 mixture and Agx@(2D-WO3). Both pure WO3 and Agx-WO3 mixture each have three well defined peaks at 2θ = 23.12°, 23.59° and 24.38°, corresponding to (002), (020) and (200) diffractions of monoclinic crystal structure with d spacing 0.385, 0.377 and 0.365 nm or interlayer spacing 0.77, 0.76 and 0.73 nm respectively. However, the Agx@(2D-WO3) core-shell samples each shows two sets of diffraction peaks: the first set is identical to what observed from the pure WO3 sample, in addition, each of the three main peaks shows a clearly resolved shoulder peak on its left side, indicating an additional phase with increased interlayer spacing. We attribute the first set to pure WO3 and the second phase the layered WO3 in the Agx@(2D-WO3) CSNS. When one side of the WO3 sheet bonds to the spherical Ag core surface, it distorts the WO3 structure, leading to the increased interlayer spacing. As the distortion would cause somewhat irregularity, it is expected that FWHM of the XRD peak would be broadened. Indeed, the (200) shoulder peak of the Agx@(2D-WO3) CSNS is widened to FWHM = 0.19 degrees, comparing to FWHM = 0.13 degrees for its main peak.
Microscopic characterization of Agx@(2D-WO3) CSNS
Transmission electron microscopy (TEM) of pure WO3, Agx@(2D-WO3) and Agx-WO3 mixture samples revealed similar layered WO3 structure, showing that the WO3 shell in the Agx@(2D-WO3) CSNS indeed maintains its layered structure around the Agx core. More specifically, Fig. 2a is a TEM image of an Ag(60nm)@(2D-WO3) CSNS, both core and shell contours are clearly displayed. For comparison, images of other samples are provided in the Supplementary Fig. S5 online. Probably due to the spherical nature of the Agx core structure, the layered WO3 shell structure was not resolved at the same degree of resolution.
TEM image and SAED pattern of pure WO3 and Ag(60nm)@(2D-WO3) CSNS.
(a), Ag(60nm)@(2D-WO3) CSNS with the core and the shell clearly resolved. The upper right inset is HRTEM image of Ag(60nm)@(2D-WO3) CSNS showing clearly resolved layered structure and the lower right inset shows the fringe spacing of 0.37 nm indexed to the (200) planes. (b), SAED pattern of the Ag(60nm) core can be indexed to standard Ag FCC crystal structure. (c), SAED pattern of the WO3 shell assigned to monoclinic structure.
To better resolve the WO3 shell structure, the Ag(60nm)@(2D-WO3) CSNS sample was cleaned using dilute ammonium solution. TEM image (upper right inset) of the sample shows clearly resolved layered structure. The spacing between adjacent layers is ~0.7–1.2 nm. As it is significantly larger than the layer spacing in the standard monoclinic WO3 structure20, we believe that it is related to the secondary phase as observed in the XRD analysis. The lower right inset shows the fringe spacing of 0.37 nm indexed to the (200) planes of monoclinic WO3.
Figure 2b, c shows selected area electron diffraction (SAED) patterns of the Ag(60nm) core and the WO3 shell. The former can be indexed to face-centered-cubic crystal structure of Ag NP with symmetry Fm3m. The latter is assigned to the WO3 monoclinic crystal with symmetry P21/n.
X-ray photoelectron spectroscopy (XPS)
The Agx@(2D-WO3) CSNS and simple Agx-WO3 mixture samples were analyzed using XPS. In order to expose fresh Ag from the samples, an ion sputtering process was used to clean them immediately before the XPS analysis. Figure 3 shows the Ag 3d XPS of Agx@(2D-WO3) CSNS and Agx-WO3 mixture. It is clear that while the Agx-WO3 mixture gives the Ag 3d5/2 and Ag 3d3/2 peaks at 368.3 eV and 374.3 eV, the peaks of Agx@(2D-WO3) CSNS shifted to 368.0 eV and 374.1 eV, respectively. As free Ag sample has the Ag 3d peaks at 368.3 eV and 374.3 eV21, no shift is found for the Agx-WO3 mixture. However, there are significant downshifts for the Agx@(2D-WO3) CSNS sample, indicating that there is an effective electron transfer from the WO3 shell to the Ag core and in other words, the Schottcky junction is formed at the Ag/WO3 interface in the CSNS, while there is no obvious Schottcky junction in the Agx-WO3 mixture.
Chemical sensor fabrication and analysis
For device fabrication (see Supplementary Fig. S1b online), a proper amount of sample (WO3, Agx-WO3 mixture, or Agx@WO3) was ground with a few drops of water in a quartz mortar to form slurry22. The slurry was then coated onto a ceramic tube equipped with an Au terminal incorporated with two Pt wire leads on each end. A resistive heating wire coil, set in the center of the tube, was employed as a heater to control sensing temperature. Sensor performance was tested on a WS-30A gas sensitivity instrument23. The sensor response (S) is defined as Ra/Rg in reducing gas atmosphere, where Ra and Rg are electrical resistance of the gas sensor in air and in testing gas atmosphere, respectively. For the test, the sensor is heated to desired temperature before Ra is measured in fresh air for 20 seconds. The test vapor is then introduced into the sensor test chamber to measure Rg as a function of time for ~60 seconds. The chamber is then opened to measure resistance until it recovers to the initial value measured in fresh air. The response and recovery time are defined as the time taken by the sensor to achieve 90% of the entire resistance change for target gas adsorption and desorption, respectively (Fig. 4).
Transient response at different temperatures for sensors using 100 ppm alcohol vapor exposure.
The upper-right inset in each figure shows corresponding response and recovery curves for the senor at its optimum working temperature. (a), pure WO3; (b), Agx-WO3 mixture; (c), Ag(25nm)@(2D-WO3); (d), Ag(35nm)@(2D-WO3); (e), Ag(45nm)@(2D-WO3); (f), Ag(60nm)@(2D-WO3).
Sensor results
The sensor response was measured with varied alcohol vapor concentration at different working temperatures. Figure 4 plots the results including sensor response, as well as response and recovery curves (as insets) for sensors using pure WO3, Agx-WO3 mixture, Ag(25nm)@(2D-WO3), Ag(35nm)@(2D-WO3), Ag(45nm)@(2D-WO3) and Ag(60nm)@(2D-WO3) towards 100 ppm alcohol vapor. It is clear that sensors made of Agx@(2D-WO3) CSNS show significantly better performance comparing to those from pure WO3 and Agx NP-WO3 mixture. For two sensors made of pure WO3 and the Agx NP-WO3 mixture, the optimum sensor working temperature is 370°C. All other sensors based on Agx@(2D-WO3) CSNS show lower optimum working temperature 340°C. Moreover, the first two sensors without core-shell structure have significantly lower responses but longer response and recovery time. Supplementary Table S1 online summarizes key sensor performance parameters. It shows that all key sensor parameters are poorer for sensors based on pure WO3 and the Agx NP-WO3 mixture, comparing to their core-shell Agx@(2D-WO3) counterparts. More specifically, they have the lowest sensor response (32 and 38 respectively), highest optimum sensor working temperature (370°C) and longest response-recovery time. Apparently, the Agx@(2D-WO3) CSNS improves all key sensor performance parameters. For example, with the CSNS, the optimum working temperature was lowered to 340°C; sensor response time was reduced to 2–4 seconds and recovery time to 5–7 seconds. The sensor made of Ag(25nm)@(2D-WO3) gives the best overall performance: response increased by ~7 times to 217 comparing to pure WO3; optimum sensor working temperature lowered to 340°C; and response and recovery time shortened to 2 and 5 seconds.
Sensor response under illumination
We measured sensor response as a function of illumination wavelength using LEDs emitting at 405 nm, 530 nm and 680 nm. For the tests, illumination intensity at the sensor was attenuated to 17 mW/cm2 for all wavelengths. Figure 5a shows the sensor response of a Ag(25nm)@(2D-WO3) CSNS based sensor at their respective optimum sensor working temperature for 100 ppm alcohol vapor. It shows that when irradiated using a 405 nm blue LED, the sensor response increases by 188% from 217 to 408, while the 530 nm and 680 nm LEDs improve the sensor performance by only ~10%.
Sensor response under illumination.
(a), Sensor response of Ag(25nm)@(2D-WO3) CSNS at different wavelength of LED irradiation. (b), Normalized sensor response for Ag(25nm)@(2D-WO3) CSNS, Agx-WO3 mixture and pure WO3 vs. light illumination intensity using a xenon arc light source (150 W) attenuated using neutral density filters. Error bars were determined from fit uncertainty in the corresponding data analysis.
We also measured sensor response as a function of light illumination intensity using a xenon arc light source (150 W) attenuated using neutral density filters. Figure 5b shows normalized response for sensors based on Ag(25nm)@(2D-WO3) CSNS, Agx-WO3 mixture and pure WO3, measured at their respective optimum sensor working temperature for 100 ppm alcohol vapor under different illumination ranging from darkness to ~1 sun intensity. For comparison, the response of the pure WO3 sensor measured at darkness is defined as 1 and all others are scaled against it. For sensors based on pure WO3 and the simple Agx-WO3 mixture, their responses increase linearly with illumination intensity. However, the increases are very limited. When light intensity increases from darkness to 87 mW/cm2, response of the pure WO3 sensor increases by only ~40% and the one with simple Agx-WO3 mixture by ~60%. The sensor made of Ag(25nm)@(2D-WO3) CSNS shows a much greater dependence on light intensity. When measured at 87 mW/cm2, response increases by ~308% comparing to its measurement in darkness.
Discussion
It is not surprising to see WO3 in the Agx@(2D-WO3) CSNS adopted its 2D layered structure. During our preparation, Agx@H2WO4 was synthesized first; it was then heated at 180°C for 24 hours for it to fully decompose into Agx@WO3. Upon another high temperature heat treatment at 500°C for 2 hours, it is expected that the WO3 shell would relax to its most stable 2D layered structure, allowing its inner peripheral to bond to the Agx core.
It is known that oxygen vacancies in WO3 lattice structure act as electron donors to provide electrons to its conduction band, making WO3 an n-type semiconductor24 and the Ag/WO3 interface a Schottky junction, as illustrated in Supplementary Fig. S3 online. As the work function of n-type WO3 is smaller than that of Ag, electrons transfer from WO3 to Ag in the Agx@(2D-WO3) CSNS, resulting in an interfacial dipole layer25. The electrically polarized potential at the interface further lowers work function of Ag and thus decreases barrier height25, leading to negatively charged Ag surface and positively charged WO3 surface or depletion zone. When the Agx@(2D-WO3) CSNS is exposed to air, it absorbs oxygen and produces negatively charged oxygen species such as Oδ−, O2δ−, etc. on its surface. As more electrons are depleted by absorbed oxygen species, the electrical resistance of the sensor (Ra) is effectively increased. On the contrary, when it is exposed to reducing atmosphere like alcohol vapor, the electron depleted WO3 surface gains electrons from the alcohol molecules, resulting in reduced electrical resistance (Rg) and therefore enlarged (Ra/Rg), leading to enhanced chemical sensor response (S = Ra/Rg).
Supplementary Fig. S4 online shows an energy band diagram for the Schottky junction formed in the Agx@(2D-WO3) CSNS. The negative XPS peak shifts for Ag 3d is caused by the electron transfer from WO3 to Ag, forming wider electron depletion layer when the sensor is exposed to air, leading to higher sensor resistance Ra. When the sensor is exposed to reducing gas such as ethanol, electrons return to WO3 conduction band due to the electron transfer between surface oxygen species and the reducing gas, resulting in reduced barrier height (ΦB) and sensor resistance Rg. The combination of higher Ra and lower Rg would yield better gas sensor response as it is defined as S = Ra/Rg.
Ag NPs act as nano antennas when they are excited by electromagnetic radiation16. In present case, our preparation process for the Agx@(2D-WO3) CSNS warrants an intimate connection between the WO3 shell and the Agx NP core. The Agx@(2D-WO3) based sensors indeed give surprisingly higher responses. We attribute the improvements to the unique combination of surface plasmonic effect of the Agx NP core, the 2D layered structure of the WO3 shell and a high quality Agx/(2D-WO3) interface. Behaving like nano antennas, Ag NPs receive excitation energy from electromagnetic radiation and transfer it via the Agx/WO3 Schottky junction to the WO3 surface, leading to collective oscillation of electron density26,27. These oscillations, known as LSPs, then lead to narrow regions of enhanced electromagnetic field strength27 and consequently improved sensor responses. The LSPs enhanced field strength in Agx NP core therefore effectively extends to the shell surface and the shell in fact behaves as an extension of LSPs from the core, leading to the changed E-field on the WO3 shell surface.
In case of Agx NP-WO3 mixture, both WO3 powder and Agx NPs were preformed separately before being mixed together. On one hand, their surfaces were saturated already by different chemical species during their syntheses. On the other hand, there must be contaminant remains in between the Agx and WO3 particles. Therefore, the spacing between Agx NP and WO3 particles are too large for effective junction formation and LSP propagation. Consequently, there is no significant improvement in sensor performance for the Agx NP-WO3 mixture.
It is also shown in Fig. 4 that within the group of Agx@(2D-WO3) based sensors, when the Agx core diameter was reduced from 60 nm to 25 nm, the smaller the Agx core diameter, the better the sensor performance. The smallest Agx core with 25 nm diameter gives the best overall performance, including highest sensor response, sharpest response and recovery time and lowest optimum sensor working temperature. This trend is anticipated considering that the ionization potential (IP) of Ag NP is expected to increase when it gets smaller due to its gradually increased electron affinity (EA). In fact, IP of Ag clusters or NPs has been studied in great detail28,29. The detailed model calculations of electronic/geometric structure exist for s1-electron metal cluster neutrals and ions in various size ranges. These calculations are found to be accurate enough for interpretation of optical response measurements on Agx30 as well as other metals31. The IPs for clusters follow the equation (1) moderately well30. Here WF is the crystalline metal work function; R the sphere radius equivalent to the volume of an x-atom metal cluster29; a the element specific extent of (cluster radius independent) electron spillout.

As EA follows the same trend as IP, when Ag NPs get smaller, both EA and IP increase and the smallest Ag NPs should have highest EA and IP. Therefore in our case, smaller Ag NPs with higher EA capture more electrons from the WO3 shell, leading to more positive space charge region on the WO3 shell surface and broadened electron depletion layer, leading to higher sensor response.
Illumination is another key factor for plasmonics. It is expected that higher illumination intensity would result in stronger LSP, leading to better sensor response. However, in order for illumination to take effect, there has to be an effective electron transfer channel between the Agx core and the WO3 shell structure.
For sensors based on pure WO3 and the simple Agx-WO3 mixture, even though their responses do increase with illumination intensity, the increased magnitudes are very limited. When light intensity increases from darkness to 87 mW/cm2, response of the pure WO3 sensor increases by only ~40% and the one with simple Agx-WO3 mixture by ~60%. The sensor made of Ag(25nm)@(2D-WO3) CSNS shows a much greater increase. When measured at 87 mW/cm2, response boosts by ~308% comparing to its measurement in darkness, revealing that there is an intimate junction between the Agx core and the 2D-WO3 shell. As for the sensor irradiated under different wavelength of LEDs, when irradiated at 405 nm, the sensor response increases by 188% (from 217 to 408). When illuminated at 530 nm and 680 nm, responses increase by only ~10%.
It is well known that Ag nanoparticles can support surface plasmons. As the resonance wavelength for the Ag(25nm) is measured at ~404 nm (see Supplementary Fig. S2 online), it is expected that the sensor response would be increased significantly at 405 nm. As the sensor response does not change much at other wavelengths, it is clear that the sensor enhancement is due to the surface plasmonic effect.
For semiconductor oxide sensors, it has been a well-known phenomenon that sensor response increases under light illumination32,33, as it is observed for the pure WO3 case. However, the increase observed for the Ag(25nm)@(2D-WO3) CSNS sensor is dramatic, suggesting that the increase is caused by the LSP effect of the Ag NP core and the enhanced E-field is effectively propagated to the 2D-WO3 shell.
To further prove the above interpretation, we conducted a Raman study to see if Ag NP can indeed effectively enhance Raman signal of the WO3 shell and surface adsorbed alcohol vapor molecules. Figure 6 shows Raman spectra of pure WO3, Agx-WO3 mixture and Agx@(2D-WO3). It is clear that each has four well-resolved peaks at 272, 326, 717 and 807 cm−1. Peaks centered at 717 and 807 cm−1 are attributed to W-O-W stretching vibration mode and two lower peaks at 272 and 326 cm−1 are induced by W-O-W bending mode vibration [(O-W-O)]34. Comparing to pure WO3, Agx-WO3 mixture shows slightly enhanced Raman intensity by only ~35%. However, Agx@(2D-WO3) CSNS shows Raman intensity enhanced by as much as 20 times, consistent with what observed from the sensor performance and further proving that LSP is a major factor for the enhanced sensor response.
In conclusion, we developed a new class of CSNS material in which 2D-WO3 was used as the shell component. We designed and fabricated a LSP enhanced chemical sensor based on Agx@(2D-WO3) CSNS. It is far more effective than sensors made of simple mixture of Ag NP and WO3 powder. It shows that the unique combination of the Ag core and the 2D layered structure of WO3 shell results in effective LSP generation and propagation, leading to much enhanced sensor performance. It is expected that more combinations of different metal core and 2D-semiconductor shell will lead to new LSP enhanced nano-devices.
Methods
Fabrication of Agx@(2D-WO3) CSNS
Size-controlled Ag NPs were synthesized using a well-developed method19. We have tested Ag NPs with diameter ranging from ~25 nm to ~60 nm, referenced by formula Ag(diameter) as Ag(25nm) and Ag(60nm). UV-Visible absorbance spectrum (see Supplementary Fig. S2 online) shows a distinct peak with center position red-shifted as Ag diameter increases. More specifically, the peaks are centered at 404 to 412, 425 and 433 nm for Ag(25nm), Ag(35nm), Ag(45nm) and Ag(60nm), respectively35. Supplementary Fig. S6 online plots the UV-Visible peak position as a function of the Ag NP diameter. Using the “Haiss equation36” and the measured λspr, the calculated diameter values agree well with measurements. As illustrated in Supplementary Fig. S1a online, the Agx@WO3 CSNS was fabricated as follows: 0.5 g Na2WO4 ·2H2O was first dissolved in 10 ml ultrapure water to prepare Na2WO4 solution. Under constant ultrasonication, 50 ml preformed Ag colloid NPs were added into the Na2WO4 solution. Note that unless specifically notified, Ag colloid concentration was adjusted to keep final atomic ratio at W/Ag = 30. Subsequently, 5 ml 5 M nitric acid was added in drop by drop under vigorous agitation. The brown suspension was turned into yellow color as H2WO4 coated onto the Ag NPs, forming Agx@H2WO4 precipitate. The mixture was then transferred into a Teflon-lined autoclave, maintained at 180°C for 24 hours. Upon cooling to room temperature, a fine yellow powder product was collected by centrifugal separation; washed using ultrapure water; dried and finally heat treated at 500°C for 2 hours. For comparison, nano Agx-WO3 mixture was prepared by simply adding preformed WO3 powder into Ag NP colloid, separating, cleaning and drying using similar procedures.
Characterization
XRD Crystal structure was characterized using a DX-2700 X-ray diffractometer (XRD) using Cu Kαradiation (λ = 0.15418 nm). The dimension and morphology of the Ag NPs were characterized using a Nova Nano SEM 450 operated at 1 kV. An UV-Vis spectroscopy (Lambda-950 spectrometer, Perkin Elmer, USA) was used to measure the absorbance spectra of Ag NPs. Elemental analysis was performed on an AXIS ULTRA X-ray photoelectron spectroscopy (XPS) instrument using monochromatic Al Kα X-ray source. TEM and SAED were taken using a JEM-2100 TEM. Raman spectroscopy study was conducted on an ALMEGA Dispersive Raman spectrometer with an Ar+ laser excitation at 514 nm. Gas sensing tests were performed using a WS-30A gas sensitivity instrument (Wei Sheng Electronics Co. Ltd., China) with a test chamber of 30 L (315 mm × 315 mm × 350 mm) in volume.
References
Bonaccorso, F., Sun, Z., Hasan, T. & Ferrari, A. C. Graphene photonics and optoelectronics. Nature Photon. 4, 611–622 (2010).
Geim, A. K. & Grigorieva, I. V. Van der Waals heterostructures. Nature 499, 419–425 (2013).
Tassin, P. Graphene for terahertz applications. Science 341, 620–621 (2013).
Qu, L., Liu, Y., Baek, J. B. & Dai, L. Nitrogen-doped graphene as efficient metal-free electrocatalyst for oxygen reduction in fuel cells. ACS Nano 4, 1321–1326 (2010).
Yu, D. & Dai, L. Self-assembled graphene/carbon nanotube hybrid films for supercapacitors. J. Phys. Chem. Lett. 1, 467–470 (2010).
Dong, H. et al. Highly sensitive multiple microRNA detection based on fluorescence quenching of graphene oxide and isothermal strand-displacement polymerase reaction. Anal. Chem. 84, 4587–4593 (2012).
Liu, Y., Yu, D., Zeng, C., Miao, Z. & Dai, L. Biocompatible graphene oxide-based glucose biosensors. Langmuir 26, 6158–6160 (2010).
Yu, D., Park, K., Durstock, M. & Dai, L. Fullerene-grafted graphene for efficient bulk heterojunction polymer photovoltaic devices. J. Phys. Chem. Lett. 2, 1113–1118 (2011).
Xie, X. et al. An asymmetrically surface-modified graphene film electrochemical actuator. ACS Nano 4, 6050–6054 (2010).
Fuhrer, M. S. & Hone, J. Measurement of mobility in dual-gated MoS2 transistors. Nature Nanotech. 8, 146–147 (2013).
Xu, M., Liang, T., Shi, M. & Chen, H. Graphene-like two-dimensional materials. Chem. Rev. 113, 3766–3798 (2013).
Butler, S. Z. et al. Progress, challenges and opportunities in two-dimensional materials beyond graphene. ACS Nano 7, 2898–2926 (2013).
Novoselov, K. S. et al. Two-dimensional atomic crystals. Proc. Natl. Acad. Sci. USA 102, 10451–10453 (2005).
Osada, M. & Sasaki, T. Two-dimensional dielectric nanosheets: novel nanoelectronics from nanocrystal building blocks. Adv. Mater. 24, 210–228 (2012).
Das, S. K. & Marsili, E. Nanomaterials-Bioinspired Metal Nanoparticle: Synthesis, Properties and Application (In Tech, 2011).
Rycenga, M. et al. Controlling the synthesis and assembly of silver nanostructures for plasmonic applications. Chem. Rev. 111, 3669–3712 (2011).
Mubeen, S. et al. An autonomous photosynthetic device in which all charge carriers derive from surface plasmons. Nature Nanotech. 8, 247–251 (2013).
Lal, S. et al. Tailoring plasmonic substrates for surface enhanced spectroscopies. Chem. Soc. Rev. 37, 898–911 (2008).
Lee, P. C. & Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 86, 3391–3395 (1982).
Liang, L. et al. High-performance flexible electrochromic device based on facile semiconductor-to-metal transition realized by WO3 ·2H2O ultrathin nanosheets. Sci. Rep. 3, 1936; 10.1038/srep01936 (2013).
Moulder, J. F., Stickle, W. F., Sobol, P. E. & Bomben, K. D. Handbook of X-ray Photoelectron Spectroscopy, Chastain, J. Ed., (Perkin Elmer Corporation, Eden Prairie, 1992).
Yin, M., Liu, M. & Liu, S. Development of an alcohol sensor based on ZnO nanorods synthesized using a scalable solvothermal method. Sensor Actuat. B-Chem. 185, 735–742 (2013).
Yin, M., Liu, M. & Liu, S. Diameter regulated ZnO nanorod synthesis and its application in gas sensor optimization. J. Alloys Compd. 586, 436–440 (2014).
Gao, X. et al. Hydrothermal synthesis of WO3 nanoplates as highly sensitive cyclohexene sensor and high-efficiency MB photocatalyst. Sensor Actuat. B-Chem. 181, 537–543 (2013).
Potje-Kamloth, K. Semiconductor junction gas sensors. Chem. Rev. 108, 367–399 (2008).
Anker, J. N. et al. Biosensing with plasmonic nanosensors. Nature Mater. 7, 442–453 (2008).
Anema, J. R., Li, J. F., Yang, Z. L., Ren, B. & Tian, Z. Q. Shell-isolated nanoparticle-enhanced Raman spectroscopy: expanding the versatility of surface-enhanced Raman scattering. Annu. Rev. Anal. Chem. 4, 129–150 (2011).
Alameddin, G., Hunter, J., Cameron, D. & Kappes, M. M. Electronic and geometric structure in silver clusters. Chem. Phys. Lett. 192, 122–128 (1992).
Seidl, M., Meiwes-Broer, K. H. & Brack, M. Finitesize effects in ionization potentials and electron affinities of metal clusters. J. Chem. Phys. 95, 1295–1303 (1991).
Rothlisberger, V. & Andreoni, W. Structural and electronic properties of sodium microclusters (n = 2–20) at low and high temperatures: new insights from ab initio molecular dynamics studies. J. Chem. Phys. 94, 8129–8151 (1991).
Rivas, L., Sanchez-Cortes, S., Garcia-Ramos, J. V. & Morcillo, G. Mixed Silver/Gold colloids: a study of their formation, morphology and surface-enhanced raman activity. Langmuir 16, 9722–9728 (2000).
Giberti, A., Malagù, C. & Guidi, V. WO3 sensing properties enhanced by UV illumination: an evidence of surface effect. Sensor Actuat. B-Chem. 165, 59–61 (2012).
Zhang, C. et al. Room temperature responses of visible-light illuminated WO3 sensors to NO2 in sub-ppm range. Sensor Actuat. B-Chem. 181, 395–401 (2013).
Su, C. Y., Lin, H. C. & Lin, C. K. Fabrication and optical properties of Ti-doped W18O49 nanorods using a modified plasma-arc gas-condensation technique. J. Vac. Sci. Technol. B 27, 2170–2174 (2009).
Li, L. et al. Controllable synthesis of monodispersed silver nanoparticles as standards for quantitative assessment of their cytotoxicity. Biomaterials 33, 1714–1721 (2012).
Pandikumar, A. & Ramaraj, R. Photocatalytic reduction of hexavalent chromium at gold nanoparticles modified titania nanotubes. Mater. Chem. Phys. 141, 629–635 (2013).
Acknowledgements
We acknowledge financial support from Chinese National University Research Fund (GK261001009), Shaanxi Normal University, Xi'an, China and China Postdoctoral Science Foundation (No.2013M542324).
Author information
Authors and Affiliations
Contributions
L.X. conducted all experimental work. L.X. and S.L. wrote the main manuscript text and M.Y. helped in interpretation of XPS, SAED data, etc. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Agx@WO3 core-shell nanostructure for LSP enhanced chemical sensors
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Xu, L., Yin, ML. & (Frank) Liu, S. Agx@WO3 core-shell nanostructure for LSP enhanced chemical sensors. Sci Rep 4, 6745 (2014). https://doi.org/10.1038/srep06745
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep06745
This article is cited by
-
Mesoporous Ag@WO3 core–shell, an investigation at different concentrated environment employing laser ablation in liquid
Scientific Reports (2024)
-
Sub-ppm of toxic gases detection on Ag-doped WO3 nanosensor
Emergent Materials (2024)
-
First-principles calculations on the structures and electronic properties of the TMW2On (TM = Mn–Ni, n = 1–6) clusters
Theoretical Chemistry Accounts (2024)
-
Photoactivation of Ag ions for improved WO3-based optoelectronic devices
Journal of Optics (2024)
-
Structures, electronic and magnetic properties of transition metal inserted W6O18 clusters
Structural Chemistry (2023)