Abstract
A field experiment was conducted using three corn cultivars (Jingyu7, Nongda80 and Tangyu10) and three nitrogen (N) application rates (0, 75 and 150 kg N ha−1). The objectives of this study were to investigate the responses of photosynthetic CO2 assimilation (Ph), the maximum quantum yield of photosystem II (Fv/Fm), leaf dry weight (LDW), leaf nitrogen concentration (LNC), leaf sugar concentration (LSC) and the sugar-to-nitrogen concentration ratio (S/N) to N levels in three different field-grown corn cultivars on three sampling dates. The results showed that the LDW, Fv/Fm, Ph, LNC and LSC increased with increasing N levels and the variation patterns of Fv/Fm, Ph and LNC were “low-high-low”. In contrast, S/N decreased with increasing N levels and its variation pattern was “high-low-high”. The values of LDW, Fv/Fm, Ph, LNC, LSC and S/N were greatest under high N conditions, followed by medium N conditions and finally low N conditions. Significant interactions occurred between Ph, Fv/Fm, LNC, LSC, LDW and S/N, with the exception of the interaction between LSC and S/N and between LSC and LDW. The correlation coefficients between Ph and S/N and between Fv/Fm and S/N were −0.714 and −0.798, respectively.
Similar content being viewed by others
Introduction
Nitrogen (N) deficiency induces changes in many physiological processes1. For example, N deficiency significantly decreases the photosynthetic CO2 assimilation capacity of leaves, leading to decreases in light-saturated photosynthetic rates2,3,4,5 and photosynthetic quantum yields6. The decrease in the photosynthetic CO2 assimilation capacity is associated with decreases in the Rubisco content and activity of RuBPcase in the Calvin cycle7.
The photosynthetic capacity of leaves and their N content are positively correlated3,8,9. Increasing N concentrations in wheat plants can be an effective method to adjust the properties of photosynthetic pigments, improve the photosystem II (PSII) potential activity and maximum quantum yield, decrease non-photochemical quenching and increase the net photosynthetic rate (Ph)10,11,12. Nitrogen deficiency decreases the quantum yield of PSII electron transport, CO2 assimilation of photosynthesis and the maximal efficiency of PSII (Fv/Fm) photochemistry13,14,15. However, other researchers have shown that N deficiency has no effect on Fv/Fm and thus results in no damage to the PSII9,16,17. For example, Nunes et al.13 showed that N deficiency had a large effect on the quantum yield of CO2 assimilation. However, Khamis et al.9 demonstrated that N deficiency had only a small effect on the quantum yield of CO2 assimilation, but it had a large effect on the light-saturated rate of photosynthesis. Nitrogen-deficient corn plants had a significantly smaller CO2 assimilation capacity, but did not differ from control plants with respect to the maximum efficiency of their PSII photochemistry (Fv/Fm)18. Zhang et al.19 suggested that the application of N fertilizer could significantly increase Fv/Fm. In their study, a high level of N fertilization increased the efficiency of the excitation energy captured by open PSII centers in certain wheat cultivar (SN1391) flag leaves. At the same time, the thermal dissipation decreased in wheat flag leaves receiving the high N treatment, but the decreased activity of the PSII resulted in the decline of the Ph in the flag leaves of certain wheat cultivars (GC8901). The results indicated that the effects of N application rates on the photosynthetic characteristics of flag leaves varied with wheat cultivars20.
In addition, studies have revealed that the total chlorophyll of leaves increases as the N supply increases21,22. Shrestha et al.23 showed that the Fv/Fm differed significantly between two or more N levels. Most previous studies on the chlorophyll fluorescence and photosynthesis of corn leaves in response to N were conducted under artificial conditions. The results from studies using artificial conditions are not truly representative of field-grown corn environments. Therefore, it is necessary to measure the chlorophyll fluorescence and photosynthesis of corn leaves in response to N and analyze the relationships between chlorophyll fluorescence, photosynthesis and leaf nitrogen concentration (LNC) in field-grown corn under different N levels. Although the sugar-to-nitrogen concentration ratio (S/N) better reflects the crop growth status than the LNC alone24, only a few field reports have investigated the relationships between chlorophyll fluorescence, photosynthesis and S/N in field-grown corn. It is important to explain the relationships between chlorophyll fluorescence, photosynthesis and S/N from the perspective of crop physiological processes, as this is useful for field corn management.
The objectives of this study were (i) to investigate Ph, Fv/Fm, LNC, leaf sugar concentration (LSC), leaf dry weight (LDW) and S/N in response to N application rates in different field-grown corn cultivars on three sampling dates and (ii) to analyze the correlations between Ph, Fv/Fm, LNC, LSC, LDW and S/N in leaves of different corn cultivars grown in the field. The results provide a practical basis for regulating N fertilizer application in corn and for breeding corn cultivars in China.
Methods
Experimental design
The field experiments were conducted from August to September during 2002 and 2003 at the Xiaotangshan experimental site (44.17°N, 116.433°E), Beijing, P.R. China. This area is representative of the overall soil and crop management in Beijing. The soil was fine-loamy with a nitrate-N (NO3-N) content of 16.7 ~ 18.03 mg kg−1, an ammonium-N (NH3-N) content of 10.2 ~ 12.3 mg kg−1, an Olsen P value of 15.2 ~ 17.6 mg kg−1, an exchangeable K content of 225 ~ 230 mg kg−1 and an organic matter content of 19.2 ~ 22.2 g kg−1 in the 0–30 cm soil layer.
Three local corn cultivars, Jingyu7 (JY7), Nongda80 (ND80) and Tangyu10 (TY10), were planted on June 25th, 2002 and June 27th, 2003. Nitrogen fertilizer (urea) was applied at three rates (0 (the low N treatment, LN), 75 (the medium N treatment, MN) and 150 (the high N treatment, HN) kg N ha−1) before planting and the N application was distributed in three splits: 50% during the seeding stage, 25% during the vegetative 6 (V6) growth stage and 25% during the vegetative tassel stage. For all treatments, 105 kg ha−1 of P2O5 (as monocalcium phosphate [Ca(H2PO4)2]) and 112.5 kg ha−1 of K2O (as KCl) were applied prior to the seeding. The size of each subplot was 15 m × 7 m. There were 2 m-wide buffer corridors between each subplot. The experiment was designed as a 2-way factorial arrangement of treatments in a randomized complete block design with three replications for each treatment. The other management elements followed the local standard practices of wheat production.
Plant measurements
The aboveground biomass was destructively sampled by randomly cutting four representative plants from each plot. All the plant samples were heated to 105°C, oven dried at 70°C until a constant weight was achieved and the samples were then weighed. The dry plant material was then ground so that it passed through a 240-mesh screen and the ground samples were analyzed for total N using a Carlo Erba NA 1500 dry combustion analyzer (Carlo Erba, Milan, Italy)36. The quantitative analysis of the total soluble sugar content was measured with the anthrone colorimetric method37.
Photosynthetic CO2 assimilation measurements
The Ph was measured with a portable leaf chamber and an open-system infrared gas analyzer (IRGA) (LI-6400; Li-Cor Inc., Lincoln, NE, USA). To minimize sources of diurnal heterogeneity, the measurements were only conducted during mid- and late- morning (usually 09:00–11:30) on uniformly sunlit days. The Ph was measured under ambient CO2 concentrations (approximately 370 μmol m−2 s−1) and a photosynthetic photon flux density (PPFD) of 1500 μmol m−2 s−1, provided by a red–blue light source (6400-02B). The ambient temperature ranged from 24 to 28°C and the temperature in the leaf chamber was about 25°C. The main function of the leaves (corn-cob leaves) was measured using the IRGA, with at least three replications. The Ph measurements were repeated five times in each subplot, for a total of five replications, which were averaged to represent the Ph of each subplot.
Chlorophyll fluorescence measurements
Chlorophyll fluorescence was determined on leaf discs using a pulse amplitude modulation portable fluorometer (PAM-2100, Walz, Effeltrich, Germany). The initial fluorescence (Fo) and maximum fluorescence (Fm) were analyzed and the Fv/Fm was calculated. The leaf discs were adapted to the dark for 30 min prior to the measurements, so that all the PSII centers were open (i.e., all the primary acceptors were oxidized) and heat dissipation was minimum. The Fo was obtained with a low-intensity modulated light (<0.1 μmol m−2 s−1), so as not to induce any effect in the fluorescence variable. The Fm was obtained with 0.3-s pulses of saturated white light with an intensity of 14,000 μmol m−2 s−1. The fluorescence variable (Fv) was calculated as the difference between Fm and Fo. The Fv and Fm values were used to obtain the Fv/Fm. The Fv/Fm was measured using the PAM-2100 while holding Ph at the same position, with at least three replications. The Fv/Fm measurements were also repeated for five replications in each subplot and were averaged to represent the Fv/Fm of each subplot.
Statistical analysis
The standard errors, variance, regression and correlation coefficients and significant differences among the regression coefficients were calculated using standard methods with SPSS software (16.0, SPSS, Chicago, IBM, USA). When it was necessary, the data was classified before the statistical analyses.
Results
Leaf dry weight (LDW)
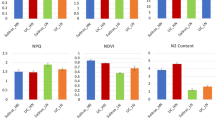
The aboveground LDW accumulation was analyzed for the three sampling dates with respect to the different N levels and corn cultivars (Figs. 1a, 1b, 1c and Table 1). We selected g m−2 (the mass of the leaves per square meter of soil) as the unit of LDW, as this unit better describes the crop growth status38. A significant difference between the different corn cultivars and N levels existed, with exception of the Nongda80 (ND80) cultivar. The results showed that the different N levels had a relatively small effect on the LDW accumulation in ND80. This indicated that the LDW accumulation in ND80 was not very sensitive to different N levels, which may be related to the wheat cultivar type. On August 16th, the LDW accumulation was greater under the HN treatment than under MN and LN treatments by 53.1% and 17.7% for the Jingyu7 (JY7) cultivar and by 60.1% and 29.0% for the Tangyu10 (TY10) cultivar, respectively. The LDW accumulation for the MN treatment was greater than that for the LN treatment by 30.1% and 24.0% for JY7 and TY10 on August 16th, respectively (Fig. 1a). Similarly, on September 6th and September 17th, the LDW accumulation was greatest under the HN treatment, followed by the MN treatment and finally the LN treatment (Figs. 1b and 1c). With the development of growth stages, the LDW accumulation changed sharply with increasing N levels for JY7 and TY10, but little change in the LDW accumulation of ND80 was observed. The effects of the N applications and cultivars were significant for the three sampling dates; however, the interactions between the N applications and cultivars were not significant (Table 1).
Leaf dry weight for August 16th (a), September 6th (b) and September 17th (c) of corn subjected to low (LN, 0 kg N ha−1), medium (MN, 75 kg N ha−1) and high (HN, 150 kg N ha−1) levels of N over the growing season (2002 and 2003).
Data are the average values across 2 years with ± one standard error (vertical bars). Analysis of variance (ANOVA) results for (a) August 16th: nitrogen (N), P < 0.004; corn cultivars (C), P < 0.045; N × C, P = 0.271; (b) September 6th: N, P < 0.002; C, P < 0.009; N × C, P = 0.145; (c) September 17th: N, P < 0.036; C, P < 0.043; N × C, P = 0.345.
The maximum quantum yield of PSII (Fv/Fm)
The different N levels had a significant effect on the seasonal dynamics of the Fv/Fm (Table 2). The JY7 and ND80 cultivars were significantly more sensitive to the different N levels than TY10. However, the effect of the N levels on TY10 was not significantly different between the HN and MN treatments, but it was significantly different between the MN and LN treatments on all three sampling dates (Tables 1 and 2). On August 16th, the Fv/Fm was higher under the HN treatment than under the MN and LN treatments by 6.8% and 4.3%, 6.7% and 3.3% and 6.2% and 2.6% for JY7, ND80 and TY10, respectively. The Fv/Fm under the MN treatment was higher than that under the LN treatment by 2.4%, 3.3% and 3.4% for JY7, ND80 and TY10 on August 16th, respectively. Similarly, the Fv/Fm was higher under the HN treatment than under the MN and LN treatments and the Fv/Fm was higher under the MN treatment than under the LN treatment on September 6th and September 17th (Tables 1 and 2). The Fv/Fm values of the different corn cultivars were significantly different on all three sampling dates and the interactions effects between the N levels and cultivars were also significant, except on September 17th (Tables 1 and 2). The results suggested that the interaction effects between the N levels and cultivars were not significant because the corn leaves entered senescence on September 17th.
Photosynthetic CO2 assimilation (Ph)
The Ph significantly increased with increasing N levels (Tables 1 and 3). On August 16th, the average Ph values under the HN treatment vs. the MN and LN treatments were 20.88 μmol m−2 s−1 vs. 26.69 μmol m−2 s−1 (+27.8%) and 33.7 μmol m−2 s−1 (+61.4%) for JY7; 18.26 μmol m−2 s−1 vs. 25.56 μmol m−2 s−1 (+40.0%) and 31.67 μmol m−2 s−1 (+73.4%) for ND80; and 17.94 μmol m−2 s−1 vs. 24.59 μmol m−2 s−1 (+37.1%) and 29.59 μmol m−2 s−1 (+64.5%) for TY10, respectively. The variations in Ph were similar to those in Fv/Fm on September 6th and September 17th (Tables 1 and 3). The N levels exhibited a strong effect on the Ph values. Similarly, the interactions between the N levels and cultivars were significant, except on September 17th (Tables 1 and 3). This was because the corn leaves entered senescence, which caused the interactions between the N levels and cultivars to be non-significant.
Leaf N concentration (mg g−1)
On August 16th, the mean LNC were 21.45 mg g−1, 29.5 mg g−1 and 36.47 mg g−1 for JY7; 21.82 mg g−1, 26.20 mg g−1 and 34.30 mg g−1 for ND80; and 19.92 mg g−1, 22.50 mg g−1 and 29.05 mg g−1 for TY10 under the LN, MN and HN treatments, respectively (Fig. 2a). On September 6th, the mean LNC increased to its maximum values, which were 37.70 mg g−1, 43.84 mg g−1 and 56.75 mg g−1 for JY7; 35.93 mg g−1, 44.36 mg g−1 and 54.26 mg g−1 for ND80; and 33.05 mg g−1, 40.73 mg g−1 and 47.12 mg g−1 for TY10 under the LN, MN and HN treatments, respectively (Fig. 2b). However, on September 17th, the mean LNC dropped sharply to 19.33 mg g−1, 28.51 mg g−1 and 32.40 mg g−1 for JY7; 21.20 mg g−1, 27.08 mg g−1 and 31.24 mg g−1 for ND80; and 16.18 mg g−1, 24.08 mg g−1 and 30.61 mg g−1 for TY10 under the LN, MN and HN treatments, respectively (Fig. 2c). The N levels significantly increased the LNC in the different corn cultivars throughout the crops' development, but the difference in the magnitude of the increase between the growth stages and corn cultivars was similar across N levels. On August 16th, the LNC was greater under the HN treatment than under the LN and MN treatments by 70.0% and 57.2%, 45.8% and 23.6% and 30.9% and 29.1%, for JY7, ND80 and TY10, respectively. In addition, the LNC was greater under the MN treatment than under the LN treatment by 37.5%, 20.8% and 12.9% for JY7, ND80 and TY10 (Fig. 2a). Similarly, on September 6th and September 17th, the LNC was greatest under the HN treatment, followed by the MN treatment and finally by the LN treatment (Figs. 2b, 2c and Table 1). The effects of the N levels and cultivars on the LNC were significant across the growth stages, but the interaction effects between the N levels and cultivars were not significant (Table 1).
Leaf N concentration on August 16th (a), September 6th (b) and September 17th (c) of corn subjected to low (LN, 0 kg N ha−1), medium (MN, 75 kg N ha−1) and high (HN, 150 kg N ha−1) levels of N application over three cropping seasons (2002 and 2003).
Data are average values across 2 years with ± one standard error (vertical bars). ANOVA results for (a) August 16th: nitrogen (N), P < 0.034; corn cultivars (C), P = 0.041; N × C, P = 0.865; (b) September 6th: N, P < 0.006; C, P = 0.035; N × C, P = 0.667; (c) September 17th: N, P < 0.041; C, P = 0.456; N × C, P = 0.766.
Leaf sugar concentration (mg g−1)
The mean LSC on September 17th was much greater than that on September 6th and August 16th. On August 16th, the mean LSC was greater than that on September 6th under the MN treatment, but less than that under the HN treatment. The LSC changed in response to N levels to a much less degree than LNC: averaged across N levels and corn cultivars, LSC increased under the MN and HN treatments by 10.8% and 18.4%, 6.83% and 20.9% and 17.2% and 33.33% on August 16th, September 6th and September 17th, respectively. The cultivar effects were not significant on the three sampling dates (Figs. 3a, 3b, 3c and Table 1). The N effects were significant for the LSC in all growth periods, but the interactions between the corn cultivars and N levels were not significant (Table 1).
Leaf sugar concentration for August 16th (a), September 6th (b) and September 17th (c) of corn subjected to low (LN, 0 kg N ha−1), medium (MN, 75 kg N ha−1) and high (HN, 150 kg N ha−1) levels of N application over the cropping season (2002 and 2003).
Data are average values across 2 years with ± one standard error (vertical bars). ANOVA results for (a) August 16th: nitrogen (N), P = 0.242; corn cultivars (C), P < 0.321; N × C, P = 0.664; (b) September 6th: N, P = 0.036; C, P < 0.431; N × C, P < 0.042; (c) September 17th: N, P = 0.032; C, P = 0.446; N × C, P = 0.862.
The sugar-to-nitrogen concentration ratio (S/N)
Exposure to N strongly decreased the S/N (Table 4) and N fertilization had a significantly positive effect on S/N (Tables 1 and 4). The changes in S/N were opposite to the changes in Ph and Fv/Fm; the S/N gradually decreased with increasing N levels and the development of growth stages (Table 4). The effects of the corn cultivars and N levels were not significant for S/N and the interactions between the N levels and corn cultivars were also not significant.
Interaction relationships between Ph, Fv/Fm, LNC, LSC, LDW and S/N
There were highly significant (P < 0.01) interactions between Ph, Fv/Fm, LNC, LSC, LDW and S/N, with the exception of the interaction between LSC and S/N and between LSC and LDW (Table 5). The results suggested that the LSC was relatively less sensitive to different N levels than LDW and S/N. Therefore, the interactions between LSC and S/N and between LSC and LDW were significant (P < 0.05).
The relationship between LDW and Fv/Fm had the highest correlation coefficient (r = 0.909), followed by that between LNC and S/N (r = −0.886). The lowest r was between LSC and LNC (r = −0.408), which were significantly (P < 0.05) correlated. The remaining results from the correlation analyses are shown in Table 5. These results indicated that Ph and Fv/Fm could be used to estimate LDW, LNC, LSC and S/N; therefore, they can serve as good indicators for monitoring LDW, LNC, LSC and S/N changes.
The LDW, Fv/Fm, Ph, LNC and LSC increased in response to increased N levels and the variation patterns of Fv/Fm, Ph and LNC were “low-high-low”. In contrast, S/N decreased as the N levels increased and its variation pattern was “high-low-high”. The results showed that the different corn cultivars and N levels significantly influenced Ph, Fv/Fm and S/N on the three sampling dates. The effects of the interactions between the N levels and cultivars on Ph, Fv/Fm, LDW, LNC and LSC were significantly different, with the exception of Ph and Fv/Fm on September 17th, but the interactions were not significantly different for S/N on the three sampling dates. Similarly, the interactions between the N levels and cultivars did not significantly affect Ph and Fv/Fm based on the analysis of the Ph and Fv/Fm values on September 17th. These results indicated that strong correlations existed between Ph and S/N and between Fv/Fm and S/N, which were −0.714 and −0.798, respectively; the relationships between LNC and Fv/Fm and between LNC and Ph were 0.636 and 0.671, respectively. This suggested that Ph and Fv/Fm could be used to estimate S/N.
Discussion
Leaf dry weight
Leaf dry weight is formed by the conversion of solar energy into biomass, a process that partially depends on the N supply25,26,27. Accordingly, in our study, LDW increased with the increased N levels and the development of growth stages. The results were in agreement with those of Biemond et al.28,29 and Vos et al.30 However, for the same N levels, the different corn cultivars also had a significant effect on LDW, probably because a large difference in N utilization and distribution existed among the cultivars. The positive relationship between leaf chlorophyll content (LCC) and LNC21,22 indicates that the solar energy absorbed by chlorophyll was converted into biomass. Because this process is related to N supply and a large difference in N utilization and distribution existed among the corn cultivars', the different LNC corresponded to the different LCC and the different LCC led to the different LDW in the different corn cultivars at the same N levels.
Ph and Fv/Fm
In this study, the Ph and Fv/Fm increased as the N levels increased (Tables 2 and 3) and these results were consistent with those of Kao et al.31 and Shang guan et al.32 However, the N applications significantly increased the Fv/Fm on the three sampling dates (Table 3), which was not in agreement with the results of Lu et al.18, but was consistent with those Zhang et al.19 and Shrestha et al.23. The former two studies suggested that the corn cultivars contained abundant chlorophyll under HN levels and thus the PSII apparatus remained functional, the crops had lots of green leaves and there was a continued use of the light captured by the remaining PSII apparatus, leading to a higher Ph, Fv/Fm, LCC and PSII efficiency. However, under LN levels, many of the green leaves became yellow and the PS II apparatus captured less light, causing lower Ph and Fv/Fm values. Under different N levels, the corn cultivars had a significant effect on the Ph and Fv/Fm, which was due to the significant effect of the corn cultivars on the LNC (Figs. 2a, 2b, 2c, Tables 2 and 3). The relatively higher LNC corresponded to the relatively higher Ph and Fv/Fm because, as some studies have shown, LCC and LNC are highly positively correlated21,22. The main reasons for this were the following: (1) LCC had a close relationship with photosynthetic electron transport, which directly affected the Ph and Fv/Fm18,19, with higher LCC resulting in higher Ph and Fv/Fm; (2) LNC strongly affected LCC23, LNC had good relationships with Ph and Fv/Fm (Table 5) and LCC and LNC were influenced by different N levels20,23; and (3) higher LCC and LNC values were linked to higher Ph and Fv/Fm values because these parameters increased the Rubisco content and activity of RuBPcase in corn plants, thereby improving corn chlorophyll fluorescence and photosynthesis. Therefore, different the N levels influenced the changes in Ph and Fv/Fm (Table 1, 2 and 3).
Leaf sugar concentration
Leaf sugar concentration (LSC) is an important indicator of physiological changes in crops. The LSC increased with increasing N levels (Figs. 3a, 3b and 3c). Hence, LNC could be used to improve the LSC in corn cultivars, which is in agreement with the findings of Sun et al.33 and Feng et al.24 However, the LSC among the different corn cultivars did not differ because the corn cultivars were less sensitive to LSC than LNC (Table 1). Over the development of growth stages, the LSC and LNC first increased gradually until the vegetation tasseling stage; then, the LSC and LNC decreased gradually until maturity, owing to the allocation of the LSC and LNC to the corn grains.
Sugar-nitrogen concentration ratio (S/N)
The effect of N on the S/N was the opposite to that of the LNC and LSC. The S/N significantly decreased with increasing N levels. The S/N significantly changed with the development of growth stages, which was consistent with the results of Tian et al.34 and the variation pattern of S/N was “high-low-high” (Table 4). The main reasons for this were as follows. (1) A positive relationship existed between LNC and LSC (Table 5). (2) Differences in the speed of LNC and LSC production existed owing to the different growth stages of corn. LSC was higher than LNC on August 16th because the corn plants were relatively small and needed more LSC to protect their normal growth from extreme environmental changes; however, over the development of its growth stages, the corn needed to increase the LNC more than the LSC for vegetation growth because the crops needed more photosynthesis to maintain their normal growth levels. Thus, the speed of LNC production was faster that of LSC and consequently, LNC was higher than LSC on September 6th. During its later growth stages, the corn required more LNC than LSC to form corn grains and maintain its photosynthetic functions and thus LSC was higher than LNC on September 17th. (3) The LNC and LSC changes were used to satisfy the needs of crop growth24 and maintain the balance of photochemistry and other physiological functions33,34. Therefore, S/N exhibited a “high-low-high” variation pattern (Table 4). The S/N was also significantly different among the different N levels (Table 1), suggesting that the different N levels also influenced the S/N levels.
Correlations between Ph, Fv/Fm, LNC, LSC, LDW and S/N
The interactions between Ph, Fv/Fm, LNC, LSC, LDW and S/N were highly significant (P < 0.01), except the interactions between LSC and S/N and between LSC and LDW. A significant relationship existed between LDW and Fv/Fm (r = 0.909), which may have been related to the strong relationship between N and Fv/Fm13,14. The main reasons for this were (1) the LCC was strongly affected by the N concentration and thus the Fv/Fm were influenced by the increased N levels19, leading to an increase in chlorophyll concentration and LDW; (2) the chlorophyll concentration and LDW also had a highly significant relationship30; (3) the increase in Fv/Fm is associated with the increase in Rubisco content and the activity of RuBPcase in the Calvin cycle and phosphoenolpyruvate carboxylase (also known as PEP carboxylase) in the C4 photosynthetic path can be used to improve the photosynthetic electron transport in chloroplasts, yielding oxygen, nicotinamide adenine dinucleotide phosphate hydrate (NADPH) and ATP, thereby increasing photosynthesis, chlorophyll concentration and thus elevating the LDW of corn plants. Therefore, the correlation coefficient between LDW and Fv/Fm was high. Similarly, a close relationship between Ph and LDW existed: the higher N concentrations increased the RuBPcase and PEP carboxylase activity, thereby improving the carbon dioxide fixation ability and increasing the Ph of corn plants; thus, the LDW increased with the increased Ph. The results also demonstrated that the LSC influenced the changes of Ph and Fv/Fm (Table 5). Strong relationships existed between the sugar concentration and Ph, Fv/Fm, LNC, LSC and LDW; this was because sugar performs a supporting role in increasing Ph, Fv/Fm, LNC, LSC and LDW. Negative correlations existed between S/N and LDW, Fv/Fm, Ph, N and S, which may be due to the fact that S/N exhibited an opposite variation pattern to LDW, Fv/Fm, Ph, LNC and LSC.
As the correlation coefficients between Ph and S/N and between Fv/Fm and S/N were −0.714 and −0.798, respectively, Ph and Fv/Fm could be used to estimate S/N (Tables 1 and 5). The correlation coefficients between LNC and Fv/Fm and between LNC and Ph were 0.636 and 0.671, respectively, which were in agreement with the results of Wu35. These results suggested that the relationships between Ph, Fv/Fm and S/N were better than those between Ph, Fv/Fm and LNC; therefore, the Ph and Fv/Fm were better estimates of S/N.
Conclusion
The results of this study showed that the LDW, Fv/Fm, Ph, LNC and LSC increased with increasing N levels in different field-grown corn cultivars on three sampling dates and the variation patterns of Fv/Fm, Ph and LNC were “low-high-low”. In contrast, S/N decreased with increasing N levels and its variation pattern was “high-low-high”. The values of LDW, Fv/Fm, Ph, LNC, LSC and S/N were the greatest under high nitrogen (HN) conditions, followed by medium nitrogen (MN) conditions and finally low nitrogen (LN) conditions. Significant (P < 0.01) interactions occurred between Ph, Fv/Fm, LNC, LSC, LDW and S/N, with the exception of the interaction between LSC and S/N and between LSC and LDW. The good correlation coefficients existed between Ph and S/N and between Fv/Fm and S/N, which were −0.714 and −0.798, respectively; the correlation coefficients between LNC and Fv/Fm and between LNC and Ph were 0.636 and 0.671, respectively.
References
McDonald, A. J. S. & Davies, W. J. Keeping in touch: response of the whole plant to deficits in water and nitrogen supply. Adv. in Bot. Res. 22, 229–300 (1996).
Wong, S. C., Cowan, I. R. & Farquhar, G. D. Leaf conductance in relation to rate of CO2 assimilation. I. Influence of nitrogen nutrition, phosphorus nutrition, photon flux density and ambient partial pressure of CO2 during ontogeny. Plant Physiol. 78, 821–825 (1985).
Sage, R. F. & Perrcy, R. W. The nitrogen use efficiency of C3 and C4 plants. II. Leaf nitrogen, effects on the gas exchange characteristics of Chenopodium album (L.) and Amaranthus retroflexus (L.). Plant Physiol. 84, 959–963 (1987).
Terashima, I. & Evans, J. R. Effects of light and nitrogen nutrition on the organization of the photosynthetic apparatus in spinach. Plant Cell Physiol. 29, 143–155 (1988).
Rascher, U., Liebig, M. & Luttge, U. Evaluation of instant light-response curves of chlorophyll fluorescence parameters obtained with a portable chlorophyll fluorometer on site in the field. Plant Cell Environ. 23, 1397–1405 (2000).
Lawlor, D. W., Boyle, F. A., Young, A. T., Keys, A. J. & Kendall, A. C. Nitrate nutrition and temperature effects on wheat: photosynthesis and photorespiration of leaves. J. Exp. Bot. 38, 393–408 (1987).
Sugiharto, B., Miyata, K., Nakamoto, H., Sasakawa, H. & Sugiyama, T. Regulation of expression of carbon-assimilating enzymes by nitrogen in maize leaf. Plant Physiol. 92, 963–969 (1990).
Ciompi, S., Gentili, E., Guidi, L. & Soldatini, G. E. The effect of nitrogen deficiency on leaf gas exchange and chlorophyll fluorescence parameters in sunflower. PlantSci. 118, 177–184 (1996).
Khamis, S., Lamaze, T., Lemoine, Y. & Foyer, C. Adaptation of the photosynthetic apparatus in maize leaves as a result of nitrogen limitation. Plant Physiol. 94, 1436–1443 (1990).
Dai, T. B., Cao, W. X. & Jing, Q. Effects of nitrogen form on nitrogen absorption and photosynthesis of different wheat genotypes. Chin. J. Appl. Eco. 12, 849–852 (2001) (in Chinese).
Dai, T. B., Cao, W. X., Sun, C. F., Jiang, D. & Jing, Q. Effect of enhanced ammonium nutrition on photosynthesis and nitrate reductase and glutamine synthetase activities of winter wheat. Chin. J. Appl. Eco. 14, 1529–1532 (2003) (in Chinese).
Fan, X. M., Jiang, D., Dai, T. B., Jing, Q. & Cao, W. X. Effects of nitrogen supply on flag leaf photosynthesis and grain starch accumulation of wheat from its anthesis to maturity under drought or waterlogging. Chin. J. Appl. Eco. 16, 1883–1888 (2005) (in Chinese).
Nunes, M. A., Ramalho, J. C. & Dias, M. A. Effect of nitrogen supply on the photosynthetic performance of leaves from coffee plants exposed to bright light. J. Exp. Bot. 262, 893–899 (1993).
Verhoeven, A. S., Demmig-Adams, B. & Adams III, W. W. Enhanced employment of the xanthophyll cycle and thermal energy dissipation in spinach exposed to high light and N stress. Plant Physiol. 113, 817–824 (1997).
Schreiber, U., Bilger, W. & Neubauer, C. [Chlorophyll florescence as anonintrusive indicator for rapid assessment of in vivo photosynthesis.] Ecophysiology of Photosynthesis [Schulze E. D., & Caldwell M. M., eds. (eds.)] [49–70] (Springer-Verlag, Berlin, 1995).
Henley, W. J., Levavasseur, G., Franklin, L. A., Osmond, B. & Ramus, J. Photoacclimation and photoinhibition in Ulva rotundata as influenced by nitrogen availability. Planta. 184, 235–243 (1991).
Bungard, R. A., McNeil, D. & Morton, J. D. Effects of nitrogen on the photosynthetic apparatus of Clematis vitalba grown at several irradiances. Aust. J. Plant Physiol. 24, 205–214 (1997).
Lu, C. M. & Zhang, J. H. Photosynthetic CO2 assimilation, chlorophyll fluorescence and photoinhibition as affected by nitrogen deficiency in maize plants. Plant Sci. 151, 135–143 (2000).
Zhang, L. M., Shangguan, P., Mao, M. C. & Yu, G. D. Effects of long-term application of nitrogen fertilization on leaf chlorophyll fluorescence of upland winter wheat. Chin. J. Appl. Eco. 14, 695–698 (2003) (in Chinese).
Cai, R. G. et al. Photosynthetic characteristics and antioxidative metabolism of flag leaves in responses to nitrogen application during grain filling of field-grown wheat. Agr. Sci. China. 7, 157–167 (2008).
Filella, I., Serrano, L., Serra, J. & Penuelas, J. Evaluating wheat nitrogen status with canopy reflectance indices and discriminant analysis. Crop Sci. 35, 1400–1405 (1995).
Moran, J. A., Mitchell, A. K., Goodmanson, G. & Stockburger, K. A. Differentiation among effects of nitrogen fertilization treatments on conifer seedlings by foliar reflectance: a comparison of methods. Tree Physiol. 20, 1113–1120 (2000).
Shrestha, S., Brueck, H. & Asch, F. Chlorophyll index, photochemical reflectance index and chlorophyll fluorescence measurements of rice leaves supplied with different N levels. J. Photochem. Photobiol. B: Biol. 113, 7–13 (2012).
Feng, W. et al. Monitoring the sugar to nitrogen ratio in wheat leaves with hyperspectral remote sensing. Acta Agron. Sin. 41, 1630–1639 (2008) (in Chinese).
Cassman, K. G., Kropff, M. J. & de Zhen, Y. A. [conceptual framework of nitrogen management of irrigated rice in high-yield environments.] Hybrid Rice Technology: New Developments and Future Prospects. [Virmani S. S., ed. (ed.)] [81–96] (International Rice Research Institute, Philippines, 1994).
Peng, S., Cassman, K. G., Virmani, S. S., Sheehy, J. & Kush, G. S. Yield potential trends of tropical rice since the release of IR 8 and the challenge of increasing rice yield potential. Crop Sci. 39, 1552–1559 (1999).
Kim, H. Y., Lieffering, M., Miura, S., Kobayashi, K. & Okada, M. Growth and nitrogen uptake of CO2 enriched rice underfield conditions. New Phytol. 150, 223–229 (2001).
Biemond, H. & Vos, J. Effects of nitrogen on the development and growth of the potato plant. 2. The partitioning of dry matter, nitrogen and nitrate. Ann. Bot. 70, 37–45 (1992).
Biemond, H., Vos, J. & Struik, P. C. Effects of nitrogen on accumulation and partitioning of dry matter and nitrogen of vegetables. 1. Brussels sprouts. Neth. J. Agric. Sci. 43, 419–433 (1995).
Vos, J., van der Putten, P. E. L. & Birch, C. J. Effect of nitrogen supply on leaf appearance, leaf growth, leaf nitrogen economy and photosynthetic capacity in maize (Zea mays L.). Field Crops Res. 93, 64–73 (2005).
Kao, W. Y., Tsai, T. T. & Chen, W. H. Responses of photosynthetic gas exchange and chlorophyll fluorescence of Miscanthus floridulus (Labill) Warb. to temperature and irradiance. J. Plant Physiol. 152, 407–412 (1998).
Shangguan, Z. P., Shao, M. G. & Dyckmans, L. Effects of nitrogen nutrition and water deficit on net photosynthetic rate and chlorophyll fluorescence in winter wheat. J. Plant Physiol. 156, 46–51 (2000).
Sun, D. Y. & Yang, J. S. The application of sugar/nitrogen in the diagnosis of wheat nutrition. Agr. Sci. China. 4, 32–39 (1974) (in Chinese).
Tian, Y. C., Zhu, Y. & Cao, W. X. Monitoring soluble sugar, total nitrogen & Its ratio in wheat leaves with canopy spectral reflectance. Acta Agron. Sin. 31, 355–360 (2005) (in Chinese).
Wu, P. Measurement of nitrogen photosynthetic rate and related leaf parameters in rice. ActaAgr. Zhejiang. 6, 131–134 (1994).
Schepers, J. S., Francis, D. D. & Thompson, M. T. Simultaneous determination of total C, total N and 15N on soil and plant material. Commun. Soil Sci. Plant Anal. 20, 949–959 (1989).
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A. & Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356 (1956).
Jin, X. L. et al. Estimation of wheat agronomic parameters using new spectral indices. PLoS ONE 8, e72736. 10.1371/journal.pone.0072736 (2013).
Acknowledgements
This study was supported by the Beijing Natural Science Foundation(4141001), the Natural Science Foundation of China (41271345), the National High Technology Research and Development Program of China (2013AA102303), the Special Funds for Technology innovation capacity building sponsored by the Beijing Academy of Agriculture and Forestry Sciences (KJCX20140417) and the Open Funds of State Key Laboratory of Remote Sensing Science, jointly sponsored by the Institute of Remote Sensing Applications of Chinese Academy of Sciences and Beijing Normal University (OFSLRSS201308).
Author information
Authors and Affiliations
Contributions
G.J.Y. and X.L.J. conceived the research, designed and performed the experiments and prepared and revised the manuscript. X.L.J. and C.W.T. analyzed the data. C.J.Z. provided technical support. All authors discussed the results and commented on the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Jin, X., Yang, G., Tan, C. et al. Effects of nitrogen stress on the photosynthetic CO2 assimilation, chlorophyll fluorescence and sugar-nitrogen ratio in corn. Sci Rep 5, 9311 (2015). https://doi.org/10.1038/srep09311
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep09311
This article is cited by
-
The effect of tillage, fertilization and residue management on winter wheat and spring wheat physiological performance
Acta Physiologiae Plantarum (2022)
-
Soybean Physiological Properties and Grain Quality Responses to Nutrients, and Predicting Nutrient Deficiency Using Chlorophyll Fluorescence
Journal of Soil Science and Plant Nutrition (2022)
-
Interactions Between Exogenous Cytokinin and Nitrogen Application Regulate Tiller Bud Growth via Sucrose and Nitrogen Allocation in Winter Wheat
Journal of Plant Growth Regulation (2021)
-
An optimization model of light intensity and nitrogen concentration coupled with yield and quality
Plant Growth Regulation (2020)
-
Low nitrogen stress regulates chlorophyll fluorescence in coordination with photosynthesis and Rubisco efficiency of rice
Physiology and Molecular Biology of Plants (2020)