Abstract
Rare-earth silicates are highly efficient materials for silicon-based light sources. Here we report a novel light-emitting device based on Ce2Si2O7. Intense violet/blue electroluminescence was observed, with a turn-on voltage of about 13 V. The violet/blue emission is attributed to 4f–5d transitions of the Ce3+ ions in Ce2Si2O7, which are formed by interfacial reaction of CeO2 and Si. Electroluminescence and photoluminescence mechanisms of the Ce2Si2O7 light-emitting device are also discussed.
Similar content being viewed by others
Introduction
Silicon-based light-emitting devices have attracted considerable attention, since silicon has many advantages such as low fabrication cost, mature fabrication and processing technology and high electrical and thermal conductivities1. In the past decades, integrations of ZnO, GaN and other III-V semiconductors with silicon were extensively studied for novel optoelectronic applications because of the direct and wide band gaps of these materrials2,3,4. However, the lattice and thermal mismatch between these materials and Si still presents a significant challenge5,6. Alternatively, rare-earth doped SiO2 has attracted a lot of interest due to their high luminescence efficiency and wide spectral range spanning from ultraviolet (UV) to infrared (IR). More importantly, SiO2 is naturally compatible with Si technology7,8. Previously, efficient visible light-emitting devices based on rare-earth doped metal-oxide-semiconductor (MOS) structures have been demonstrated9,10,11. However, the solubility of rare-earth ions in Si-based materials achieved so far is still relatively low (1016−1020 cm−3)12. In fact, even if the solubility could be improved, since the rare-earth ions (e.g. Er3+, Eu3+, Ce3+) are optically inactive at high concentrations due to the formation of clusters13,14,15, such a concentration quenching would still be a significant barrier for further improving performance of rare-earth doped Si-based materials. Recently, it was shown that formation of rare-earth silicates (e.g. Er2SiO5, Eu2SiO5, Ce2Si2O7) could enhance their photoluminescence efficiency16,17,18. It was found that in these silicates, the rare-earth ions are 100% activated and the solubility can reach 1022 cm−3 19,20,21.
Ce2Si2O7 is a promising material for UV optoelectronic devices, with an emission band similar to that of ZnO and GaN. In addition, different emission wavelengths could be obtained from the energy transfer between Ce3+ and other rare-earth ions (such as Er3+, Tb3+, Dy3+, Sm3+)10,11,22,23. Although photoluminescence (PL) of Ce2Si2O7 has been investigated by several groups16,18,24,25, there has been no reports on electroluminescence (EL) of Ce2Si2O7 so far. In this paper, Ce2Si2O7 is synthesized by interfacial reaction of CeO2 thin film and Si wafer in reducing ambient. Violet/blue emissions at about 390 and 410 nm from Ce2Si2O7 were obtained. The internal quantum efficiency of Ce2Si2O7 was found to be about 37% at room temperature. Finally, we fabricated Ce2Si2O7 light-emitting devices (LEDs) and achieved a turn on voltage of 13 V, while the maximum EL intensity was obtained at a driving voltage of 25 V.
Results and Discussion
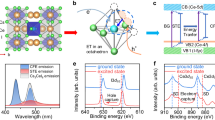
In order to investigate the effect of annealing ambient on the structural change of CeO2 films, X-ray diffraction (XRD) patterns of as-deposited and annealed CeO2 films were measured. As shown in Fig. 1(a), the crystal structure of the samples depends strongly on the annealing ambient. The as-deposited film contains a broad reflection around 28.6°, which corresponds to the (111) plane of CeO2. When the as-deposited film was annealed in O2 ambient, the diffraction peak at 28.6° becomes higher and narrower and the (200) plane of CeO2 can be identified. The Ce2Si2O7 phase is obtained after the as-deposited CeO2 film was annealed in reducing ambient and the highest reflection peak is identified as the (008) plane. The lattice structure is also investigated by transmission electron microscopy (TEM). As shown in Fig. 1(b), the thickness of Ce2Si2O7 is about 50 nm. There is an amorphous SiOx layer at the Ce2Si2O7/Si interface, with a thickness of about 5 nm. During the annealing process, CeO2 was reduced to CeO2−x, while Si was oxidized. Then, SiOx and CeO2−x reacted at high temperature, resulting in Ce2Si2O7 with various crystallographic orientations.
The lattice structure of Ce2Si2O7 observed is different from previous reports18,26,27. We attribute this to the strong dependence of the crystallinity and orientation on deposition and annealing conditions27,28,29. To confirm this, the as-deposited CeO2 films with different thicknesses were annealed in reducing ambient (Ar:H2 = 97:3) for 1 hour. As shown in Fig. 2(a), there are various crystallographic orientations of Ce2Si2O7 when 300 nm CeO2 film was annealed at 1020 °C. However, the intensity of the (008) diffraction peak increases with decreasing the thickness of CeO2. In Fig. 2(b), various crystallographic orientations of Ce2Si2O7 are exhibited when a 70 nm CeO2 film was annealed at 1000 °C. Unlike the 300 nm film, here the (008) diffraction peak initially increases with the annealing temperature up to 1020 °C and then decreases while the annealing temperature is further raised to above 1060 °C. Similarly, as shown in Fig. 2(c), this peak first increases with the annealing ambient flow rate is increased and then decreases once the flow rate up is above 5000 sccm. In summary, these observations show that the deposition and annealing conditions are critical factors for synthesizing Ce2Si2O7.
Figure 3 shows the PL spectrum of the CeO2 film annealed in Ar:H2 ambient. The intense violet/blue emission consists of two peaks centered at about 390 nm (3.18 eV) and 410 nm (3.02 eV), respectively. Both the shape and the position of the peaks are similar to the results obtained by Choi18. However, these emission bands are not observed in the as-deposited films or the samples annealed in O2 ambient. Combined with crystal structure analysis mentioned above, the violet/blue emission bands are attributed to the formation of Ce2Si2O7 and correspond to the Ce3+ transitions from the relaxed lowest 5d excited state to 4f ground states. The energy separation of the two peaks is about 2000 cm−1, which matches well with the theoretical value of spin–orbit splitting between the 4f ground state 2F5/2 and 2F7/2 30. The excitation bands located at 295 nm (4.2 eV) and 324 nm (3.83 eV) can be assigned to the 4f–5d transitions31. Based on these assignments, a simplified energy level diagram is sketched in Fig. 3. In order to estimate the optical band gap (Egopt) of Ce2Si2O7, we measured the absorption spectrum of the sample in the visible range and obtain a value of 3.6 eV by extrapolating the spectrum using Tauc’s relation32:

where α is the absorption coefficient, h is the Planck’s constant, ν is the photon frequency and C is a constant.
According to several previous studies, Ce3+ activator concentration is limited to about 0.5 at% in most host materials33,34,35. However, our results show that Ce3+ ions in Ce2Si2O7 are almost 100% activated. This is similar to self-activated materials such as CeF3 and CeCl3 36. We find that Ce3+ emission is enhanced significantly by the formation of Ce2Si2O7. To quantify this enhancement, we measured the internal quantum efficiency of Ce2Si2O7 by using an integrated sphere37. We obtained a value of about 37% at room temperature. To reveal the excitation and emission mechanisms, we measured the PL spectra as a function of temperature. As shown in Fig. 4, the PL intensity increases as the sample temperature is decreased. We also measured PL decay time at temperatures of 77 and 300 K, respectively, as shown in the inset of Fig. 4. The PL decays exponentially, with the time constant slightly changes from 17 to 21 ns as the temperature is decreased from 300 to 77 K. This very short lifetime is in agreement with the electric-dipole allowed 4f–5d transition rate of Ce3+. Since non-radiative centers are frozen at low temperatures, we attribute the decrease of the PL intensity and the decay time to the energy transfer to defect centers by non-radiative recombination processes38.
Next, we fabricated Ce2Si2O7 LEDs with the structure diagram shown in Fig. 5. Violet/blue EL emission is clearly observed when a positive voltage is applied on the indium tin oxide (ITO) layer. The turn-on voltage of the device is found to be as low as 13 V. The EL spectra (Fig. 5) are in good agreement with the PL spectra (Fig. 3). The EL intensity increases with the forward voltage and the maximum EL intensity is obtained at 25 V. Figure 6 presents a typical current-voltage (I-V) characteristics. The forward current of the device reaches up to 0.26 mA when the forward bias is 25 V, while a reverse leakage current of 6.4 μA at a bias voltage of −10 V is observed. These values illustrate the excellent rectification performance achieved in this device.
Based on the energy level diagram of Ce2Si2O7 developed in this work, we propose the following mechanism of the LED operation: As seen from the inset of Fig. 6, an asymmetrical energy barrier is formed at the junction interface. When a positive voltage is applied between the two electrodes (forward bias), the electrons accumulated in the Si/SiOx interface are swept to Ce2Si2O7 side by tunneling through the SiOx barrier. Meanwhile, the holes are injected into Ce2Si2O7 from ITO and accumulated at Ce2Si2O7/SiOx interface due to the SiOx barrier. As a result, the EL originates from the recombination between the injected electrons and holes in Ce2Si2O7. These emitted photons with an energy (hυ) approximately equals to the energy difference between 4f and 5d states of Ce3+. Hence, in this design, the SiOx layer functions as a carrier blocking layer. For high-performance and reliable device operation, the ideal thickness of SiOx layer should be less than 2 nm39. Therefore, the turn-on voltage can be further decreases, considering the 5 nm SiOx layer used in this device.
In summary, a new method of synthesizing Ce2Si2O7 was demonstrated. Ce2Si2O7 was formed after the Si-based CeO2 film was annealed in reducing ambient. Intense violet/blue emission was observed from Ce2Si2O7 and the PL emission bands are located around 390 and 410 nm, which are attributed to 4f–5d transitions of Ce3+. More importantly, Ce2Si2O7 LEDs were fabricated and strong violet/blue EL emission was observed. The turn on voltage of Ce2Si2O7 LED is 13 V and the maximum EL intensity was obtained at 25 V.
Methods
CeO2 thin films were deposited on P-type Si (100) substrates by electron beam evaporation (EVA 450). The as-deposited films were annealed in reducing ambient (Ar:H2 = 97:3) at various temperatures for 1 hour. We fabricated the LEDs as schematically illustrated in the inset of Fig. 5. ITO and Ag electrodes were deposited on the surface of the film and the back side of Si substrates, respectively, both by magnetron sputtering. The PL spectra were measured by a He-Cd laser with a 325 nm excitation wavelength and the PL excitation (PLE) spectrum was measured with a fluorescence spectrometer (FLS920) using a 450 W xenon lamp as the excitation source. The crystal structures were characterized by XRD using Cu Kα radiation (Bruker D8 ADVANCE) and morphology of the samples was determined by TEM (Hitachi, H8100 200 kV). The internal quantum efficiency of samples is measured using an F-3018 integrating sphere with a 335 nm laser excitation. The PL decay was measured by using a nanosecond xenon flash lamp at 325 nm excitation wavelength and detected by a time-correlated single photon-counting system. The EL spectra of the devices and I-V characteristics were measured by a system of an ACTON 150 CCD spectrometer and a Keithley 2410, respectively.
Additional Information
How to cite this article: Li, L. et al. A novel violet/blue light-emitting device based on Ce2Si2O7. Sci. Rep. 5, 16659; doi: 10.1038/srep16659 (2015).
References
Hirschman, K. D., Tsybeskov, L., Duttaguptap, S. P. & Fauchet, M. Silicon-based visible light-emitting devices integrated into microelectronic circuits. Nature 384, 338–341 (1996).
Nguyen, H. P. T. et al. p-Type Modulation Doped InGaN/GaN Dot-in-a-Wire White-Light-Emitting Diodes Monolithically Grown on Si(111). Nano Lett. 11, 1919–1924 (2011).
Hsieh, Y. P. et al. Electroluminescence from ZnO/Si-nanotips light-emitting diodes. Nano Lett. 9, 1839–1843 (2009).
Ra, Y. H., Navamathavan, R., Park, J. H. & Lee, C. R. High-Quality Uniaxial InxGa1−xN/GaN Multiple Quantum Well (MQW) Nanowires (NWs) on Si(111) Grown by Metal-Organic Chemical Vapor Deposition (MOCVD) and Light-Emitting Diode (LED) Fabrication. ACS Appl. Mater. Interfaces 5, 2111–2117 (2013).
Xiong, C. et al. Integrated GaN photonic circuits on silicon (100) for second harmonic generation. Opt. Express 19, 10462–10470 (2011).
Triviño, N. V. et al. Integrated photonics on silicon with wide bandgap GaN semiconductor. Appl. Phys. Lett. 102, 081120 (2013).
Rebohle, L. et al. Strong electroluminescence from SiO2-Tb2O3-Al2O3 mixed layers fabricated by atomic layer deposition. Appl. Phys. Lett. 104, 251113 (2014).
Izeddin, I., Moskalenko, A. S., Yassievich, I. N., Fujii, M. & Gregorkiewicz, T. Nanosecond Dynamics of the Near-Infrared Photoluminescence of Er-Doped SiO2 Sensitized with Si Nanocrystals. Phys. Rev. Lett. 97, 207401 (2006).
Sun, J. M. et al. Efficient ultraviolet electroluminescence from a Gd-implanted silicon metal-oxide-semiconductor device. Appl. Phys. Lett. 85, 3387–3389 (2004).
Sun, J. M., Skorupa, W., Dekorsy, T., Helm, M. & Nazarov, A. N. The effect of annealing conditions on the crystallization of Er-Si-O formed by solid phase reaction. Opt. Mater. 27, 1050–1054 (2005).
Sun, J. M. et al. Bright green electroluminescence from Tb3+ in silicon metal-oxide-semiconductor devices. J. Appl. Phys. 97, 123513 (2005).
Polman, A. et al. A. Erbium in crystal silicon limits. J. Appt. Phys. 77, 1256–1262 (1995).
Watras, A., Dereń, P. J. & Pąik, R. Luminescence properties and determination of optimal RE3+ (Sm3+, Tb3+ and Dy3+) doping levels in the KYP2O7 host lattice obtained by combustion synthesis. New J. Chem. 38, 5058–5068 (2014).
Hori, Y. et al. GaN quantum dots doped with Tb. Appl. Phys. Lett. 88, 053102 (2006).
Xia, Z. G. & Liu, R. S. Tunable Blue-Green Color Emission and Energy Transfer of Ca2Al3O6F:Ce3+, Tb3+ Phosphors for Near-UV White LEDs. J. Phys. Chem. C 116, 15604–15609 (2012).
Choi, H. J. et al. Self-Organized Growth of Si/Silica/Er2Si2O7 Core-Shell Nanowire Heterostructures and their Luminescence. Nano Lett. 5, 2432–2437 (2005).
Savio, R. L. et al. Enhanced 1.54 μm emission in Y-Er disilicate thin films on silicon photonic crystal cavities. Opt. Express 21, 10278–10288 (2013).
Choi, W. C. et al. Violet/blue light-emitting cerium silicates. Appl. Phys. Lett. 75, 2389–2391 (1999).
Miritello, M. et al. Efficient Luminescence and Energy Transfer in Erbium Silicate Thin Films. Adv. Mater. 19, 1582–1588 (2007).
Masaki, K., Isshiki, H., Kawaguchi, T. & Kimura, T. The effect of annealing conditions on the crystallization of Er-Si-O formed by solid phase reaction. Opt. Mater. 28, 831–835 (2006).
Isshiki, H., Dood, M. J. A., Polman, A. & Kimura, T. Self-assembled infrared-luminescent Er-Si-O crystallites on silicon. Appl. Phys. Lett. 85, 4343–4345 (2004).
Li, L. et al. Investigation on white light emissions from CeO2/Dy2O3 multilayer films based on silicon substrates. Vacuum 112, 38–41 (2015).
Li, L. et al. Investigation on photoluminescence properties of CeO2/Sm2O3 multilayer films based on Si substrates. Phys. Status Solidi B. 251, 737–740 (2014).
Kepinski, L., Hreniak, D. & Strek, W. Microstructure and luminescence properties of nanocrystalline cerium silicates. J. Alloy Compd. 341, 203–207 (2002).
Li, J. et al. The formation of light emitting cerium silicates in cerium-doped silicon oxides. Appl. Phys. Lett. 94, 011112 (2009).
Quah, H. J. et al. Effects of Postdeposition Annealing in Argon Ambient on Metallorganic Decomposed CeO2 Gate Spin Coated on Silicon. J. Electrochem Soc. 157, 6–12 (2010).
Kepinski, L., Wolcyrz, M. & Marchewka, M. Structure Evolution of Nanocrystalline CeO2 Supported on Silica: Effect of Temperature and Atmosphere. J. Solid State Chem. 168, 110–118 (2002).
Mamatrishat, M. et al. Valence number transition and silicate formation of cerium oxide films on Si(100). Vacuum 86, 1513–1516 (2012).
Pagliuca, F., Luches, P. & Valeri, S. Interfacial interaction between cerium oxide and silicon surfaces. Surf. Sci. 607, 164–169 (2013).
Dieke, G. H. Spectra and Energy Levels of Rare Earth Ions in Crystals (Plenum Publications, New York, 1968).
Skorodumova, N. V. et al. Electronic, bonding and optical properties of CeO2 and Ce2O3 from first principle. Phys. Rev. B 64, 115108 (2001).
Tauc, J., Grigorovici, R. & Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Stat. Sol. 15, 627–637 (1966).
Koao, L. F., Swart, H. C., Obed, R. I. & Dejene, F. B. Synthesis and characterization of Ce3+ doped silica (SiO2) nanoparticles. J. Lumin. 131, 1249–1254 (2011).
Katelnikovas, A. et al. Photoluminescence in sol-gel-derived YAG:Ce phosphors. J. Cryst. Growth 304, 361–368 (2007).
Feng, H., Ding, D. Z. & Li, H. Cerium concentration and temperature dependence of the luminescence of Lu2Si2O7:Ce scintillator. J. Alloy Compd. 509, 3855–3858 (2011).
Li, Y. & Yan, B. Functionalized Mesoporous SBA-15 with CeF3: Eu3+ Nanoparticle by Three Different Methods: Synthesis, Characterization and Photoluminescence. Nanoscale Res. Lett. 5, 701–708 (2010).
Mello, J. C., Wittmann, H. F. & Friend, R. H. An improved experimental determination of external photoluminescence quantum efficiency. Adv. Mater. 9, 230–232 (1997).
Lee, B. G. et al. Strained Interface Defects in Silicon Nanocrystals. Adv. Funct. Mater. 22, 3223–3232 (2012).
Alers, G. B. et al. Intermixing at the tantalum oxide/silicon interface in gate dielectric structures. Appl. Phys. Lett. 73, 1517–1519 (1998).
Acknowledgements
This work was financially supported by the National Science Foundation of China (Grant no. 60977017, 61275058) and the Fundamental Research Funds for the Central Universities (2013JBM101).
Author information
Authors and Affiliations
Contributions
L.X.Y. conceived the idea and designed the study. L.L. carried out experiments and wrote the manuscript. L.L., S.W.W., G.Y.M., X.Y., K.O. and L.X.Y. discussed the results and commented the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Li, L., Wang, S., Mu, G. et al. A novel violet/blue light-emitting device based on Ce2Si2O7. Sci Rep 5, 16659 (2015). https://doi.org/10.1038/srep16659
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep16659
This article is cited by
-
Synthesis of CeO2-reduced graphene oxide nanocomposite for display and latent fingerprint application
Journal of Materials Science: Materials in Electronics (2024)
-
Influence of cerium content and heat treatment on Ce:YAG@glass wool nanostructures
Journal of Nanoparticle Research (2019)
-
Green electroluminescence from Tb2O3/polymer heterojunction light-emitting diodes
Journal of Materials Science (2018)
-
Multicolor light-emitting devices with Tb2O3 on silicon
Scientific Reports (2017)
-
Fabricated CeO2 nanopowders as a novel sensing platform for advanced forensic, electrochemical and photocatalytic applications
Applied Nanoscience (2017)