Abstract
Dysregulation of emotional arousal is observed in many psychiatric diseases such as schizophrenia, mood and anxiety disorders. The neurotrophic tyrosine kinase receptor type 2 gene (NTRK2) has been associated with these disorders. Here we investigated the relation between genetic variability of NTRK2 and emotional arousal in healthy young subjects in two independent samples (n1=1171; n2=707). In addition, diffusion tensor imaging (DTI) data in a subgroup of 342 participants were used to identify NTRK2-related white-matter structure differences. After correction for multiple testing, we identified a NTRK2 single nucleotide polymorphism associated with emotional arousal in both samples (n1: Pnominal=0.0003, Pcorrected=0.048; n2: Pnominal=0.0141, Pcorrected=0.036). DTI revealed significant, whole-brain corrected correlations between emotional arousal and brain white-matter mean diffusivity (MD), as well as significant, whole-brain corrected NTRK2 genotype-related differences in MD (PFWE<0.05). Our study demonstrates that genetic variability of NTRK2, a susceptibility gene for psychiatric disorders, is related to emotional arousal and—independently—to brain white-matter properties in healthy individuals.
Similar content being viewed by others
Introduction
A dysregulation of emotional processes is commonly observed in many psychiatric disorders. Specifically, more than 75% of psychiatric diagnostic categories are characterized by emotional dysregulation.1 In some psychiatric disorders, like mood or anxiety disorders, emotional dysregulation is one of the core symptoms giving rise to the respective diagnosis.1
The International Affective Picture System (IAPS),2 which consists of a large set of standardized, emotion-evoking photographs covering a wide range of semantic categories, is often used in experimental studies to test and quantify emotional processes. Typically, subjects are asked to rate these pictures on two emotion dimensions, namely valence (ranging from pleasant to unpleasant) and arousal (ranging from calm to excited).2 Differences in ratings of IAPS pictures have been observed in patients with psychiatric disorders.3 Several studies demonstrated altered arousal ratings of IAPS pictures in schizophrenia, borderline personality disorder, major depressive disorder and substance abuse.4, 5, 6, 7, 8, 9
Heritability of psychiatric disorders is generally high with heritability estimates ranging between 40 and 80%,10, 11, 12, 13, 14, 15 suggesting substantial genetic contribution to disease risk. The gene encoding the neurotrophic tyrosine kinase receptor type 2 (NTRK2 also known as TRKB) has been often discussed in this context, mostly due to its involvement in the neurotrophic hypothesis of stress-related psychiatric disorders.16, 17 NTRK2 can be activated by several neurotrophins including brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT3) and NT4/5.18, 19, 20, 21 TrkB signaling triggered by neurotrophins plays a central role in cell proliferation, survival and differentiation, as well as synaptic plasticity, and neurotransmitter release.22, 23, 24, 25, 26 Specifically, NTRK2, which activates several intracellular signaling cascades on ligand binding, is known to interact with expression and trafficking of glutamate receptors.26, 27 Importantly, NTRK2 modulates specific phases of fear learning and synaptic plasticity in the amygdala,28 a brain region centrally involved in the processing of emotionally arousing stimuli and in the pathogenesis of psychiatric conditions.29, 30 Several recent studies have indeed suggested a relationship between NTRK2 and a broad range of psychiatric disorders such as depression, schizophrenia, addiction, eating and anxiety disorders.31, 32, 33, 34, 35, 36, 37, 38, 39, 40 For example, Ernst et al.33 identified a deletion in a human NTRK2 promoter and provided evidence for an involvement of this deletion in anxiety traits. In two independent samples of depressed patients, Kohli et al.37 observed an association between lifetime history of suicide attempts and a combination of several independent risk alleles within the NTRK2 gene. In addition, a study also points to an involvement of genetic NTRK2 variants in brain imaging parameters, such as genotype-dependent differences in white-matter properties in depressed patients.41 In support of the genetic data, the expression pattern of NTRK2 in the brain, with high expression levels in different brain regions such as the occipital, temporal and frontal cerebral cortices, the putamen and the cerebellar cortex,18, 42, 43 also argues against a role for NTRK2 in a specific psychiatric disorder. The available data rather point to an involvement of NTRK2 in general mental processes underlying psychopathology.
In summary, NTRK2 modulates glutamate receptor function and synaptic plasticity in the amygdala, which is centrally implicated in emotional arousal in health and mental disease. Thus, we hypothesized that NTRK2 genetic variants are associated with emotional arousal independently of disease status. Specifically, we quantified the arousal ratings of emotional (negative and positive) and neutral IAPS pictures in two independent samples of healthy young subjects (n1=1171, n2=707). Since genetic variants in genes encoding for neurotrophic receptors have been associated with white-matter measures,44, 45 we analyzed in a subsample of 342 subjects white-matter properties as assessed by diffusion tensor imaging (DTI) in relation to arousal ratings, as well as NTRK2 genotype. In this study, we focused on (a) fractional anisotropy (FA), which is a measure of the directional dependence of diffusion46 and reflects fiber density, as well as coherence within a voxel,47 and (b) mean diffusivity (MD), which reflects the magnitude of water diffusion within a voxel and depends on the density of physical obstructions like membranes and the distribution of water molecules between different cellular compartments.47, 48
Materials and methods
Participants
In total, we analyzed data of N=1878 subjects from 2 independent samples, that is, a hypothesis-testing (Basel_1) and a hypothesis-confirming sample (Basel_2). Overall, 65% of the subjects were female and the mean age was 23±3 years (age-range 18–35; for information about each sample separately see Supplementary Table S1). Subjects were recruited from the area of Basel in Switzerland. Sampling strategy was to recruit large samples of healthy young adults, without further restrictions. Advertising was done mainly in the University of Basel. Subjects were free of any neurological or psychiatric illness, and did not take any medication at the time of the experiment. The ethics committee of the Cantons of Basel City and Basel Country approved the experiments. Written informed consent was obtained from all subjects prior to participation.
Behavioral tasks
All subjects performed an identical picture task. This task consisted of the presentation of 24 IAPS pictures per valence group (negative, neutral and positive), as well as pictures from in-house standardized picture sets. Subjects rated the presented pictures according to valence (positive=1, neutral=0, negative=−1) and arousal (high=3, medium=2, low=1). For more detailed sample task and procedure description see Supplementary I paragraph Procedure and task description.
Saliva DNA samples collection and isolation
Saliva samples were collected from all subjects at the time-point of the main investigation, using Oragene DNA Kit (DNA Genotek, Ottawa, ON, Canada). Saliva DNA was extracted from the Oragene DNA Kit using the standard precipitation protocol recommended by the producer.
Array-based SNP genotyping
Samples were processed as described in the Genome-Wide Human SNP Nsp/Sty 6.0 User Guide (Affymetrix, Santa Clara, CA, USA). Briefly, genomic DNA concentration was determined by using a Nano-Drop ND-1000 and adjusted to 50 ng ml−1 in water; 250 ng of DNA was digested in parallel with 10 U of StyI and NspI restriction enzymes (New England Biolabs, Ipswich, MA, USA) for 2 h at 37 °C. Enzyme-specific adaptor oligonucleotides were then ligated onto the digested ends with T4 DNA Ligase for 3 h at 16 °C. After adjustment to 100 ml with water, 10 ml of the diluted ligation reactions were subjected to PCR. Three PCR reactions of 100 ml were performed for Sty-digested products and four PCR reactions for Nsp. PCR was performed with Titanium Taq DNA Polymerase (Clontech, Mountain View, CA, USA) in the presence of 4.5 mm PCR primer 002 (Affymetrix), 350 mm each dNTP (Clontech), 1 m G-C Melt (Clontech) and 13 Titanium Taq PCR Buffer. Cycling parameters were as follows: initial denaturation at 94 °C for 3 min, amplification at 94 °C for 30 s, 60 °C for 45 s and extension at 68 °C for 15 s repeated a total of 30 times, final extension at 68 °C for 7 min. Reactions were then verified to migrate at an average size between 200–1100 bp using 2% TBE gel electrophoresis. PCR products were combined and purified with the Filter Bottom Plate (Seahorse Bioscience, North Billerica, MA, USA) using Agencourt Magnetic Beads (Beckman Coulter, Brea, CA, USA). Purified PCR products were quantified on a Zenith 200rt microplate reader (Anthos-Labtec, Salzburg, Austria). We obtained 4–5 mg ml−1 on average for each sample. From this stage on, the SNP Nsp/Sty 5.0/6.0 Assay Kit (Affymetrix) was used. Around 250 mg of purified PCR products were fragmented using 0.5 U of DNase I at 37 °C for 35 min. Fragmentation of the products to an average size <180 bp was verified using 4% TBE gel electrophoresis. After fragmentation, the DNA was end labeled with 105 U of terminal deoxynucleotidyl transferase at 37 °C for 4 h. The labeled DNA was then hybridized onto Genome-Wide Human SNP 6.0 Array at 50 °C for 18 h at 60 r.p.m. The hybridized array was washed, stained and scanned according to the manufacturer’s (Affymetrix) instructions using Affymetrix GeneChip Command Console (AGCC, version 3.0.1.1214, Affymetrix). Generation of single nucleotide polymorphisms (SNP) calls and Array quality control (QC) were performed using the command line programs of the Affymetrix Power Tools package (version: apt-1-14.4.1, Affymetrix). According to the manufacturer’s recommendation, contrast QC was chosen as QC metric, using the default value of ⩾0.4. All samples passing QC criteria were subsequently genotyped using the Birdseed (v2) algorithm (Broad Institute, Cambridge, MA, USA). Mean Call Rate for all samples averaged >98.4%. This value refers to per sample (that is, individual) call rate and ranged from 93 to 100%.
SNP selection
Generation of SNP calls and array QC were performed using the Affymetrix Genotyping Console Software 3.0 (Affymetrix). Genotypic outliers were identified and removed using Bayesian clustering algorithm49 (for more details see Supplementary II paragraph Sample QC with Bayesian clustering algorithm).
SNPs on the NTRK2 gene (50 kb upstream and 10 kb downstream, chr9:87,233,466-87,648,505) based on University of California Santa Cruz (UCSC, http://genome.ucsc.edu) annotation hg19 were used for analysis. For association testing, markers with genotype call rate <0.98, with minor allele frequency <0.01 and with Hardy–Weinberg equilibrium P<0.05 were excluded leading to a reduction from 135 markers to 85 markers to be analyzed.
Genetic association analysis
Analyses of variance were calculated with the WG-Permer software (www.wg-permer.org). This software corrects nominal P-values for multiple testing on a permutation-based procedure (number of permutations set to 10 000) according to the study by Westfall and Young.50 Dominant and recessive models of inheritance were calculated.
Mean arousal ratings per valence category (positive, negative and neutral) of IAPS pictures served as quantitative phenotypes. Since sex- and age-related differences in emotional processing are observed,51, 52 we corrected for these variables by using the z-transformed residuals derived from a linear regression.
In the hypothesis-testing sample, behavioral data for the phenotypes of interest were available for 1552 subjects, of whom 1389 subjects had genetic data. After QC, we excluded 160 subjects (genetic background outliers: 154 subjects, gender inconsistencies: 6 subjects). In total 1171 subjects had a complete data set (behavioral and genetic data). The hypothesis-confirming sample comprised initially 1024 subjects with behavioral data on the phenotypes of interest. Of these 1024 subjects, 827 subjects had genetic data. After QC, we excluded 104 subjects (genetic background outliers: 97 subjects, gender inconsistencies: 7 subjects). In total, 707 subjects had a complete data set (behavioral and genetic data).
In addition to the candidate gene analysis, a genome-wide association study of emotional arousal was performed with the array data. PLINK53 was used for association testing under the dominant, recessive and additive genetic model. SNPs with a genotype call rate <0.99, a minor allele frequency <0.01 and with a Hardy–Weinberg equilibrium P<0.001 were excluded. Only Bonferroni-corrected P-values <5 × 10−8 were considered significant.
DTI data acquisition and analysis
Diffusion volumes were acquired on a 3 T scanner using a single-shot echo-planar sequence, and consisted of 64 diffusion-weighted volumes (b=900 s.mm−2) and 1 unweighted volume (b=0 s.mm−2). Diffusion data were analyzed using FMRIB Software Library v4.1.7 (FSL, http://www.fmrib.ox.ac.uk/fsl). Maps of FA and MD were obtained from the diffusion tensor (DT) model for further analyses. Voxel-wise statistical analyses of FA and MD maps were carried out using the Tract-Based Spatial Statistics (TBSS) toolbox of FSL.54 Voxel-wise statistical analyses were run on the skeletonized FA and MD maps using permutation-based nonparametric inference within the framework of the general linear model55 as implemented in the randomise tool.56 Five thousand permutations were performed and results were considered significant for P<0.05, corrected for multiple comparisons across space using the 'two-dimensional' parameter settings with threshold-free cluster enhancement, which avoids using an arbitrary threshold for the initial cluster-formation.57 Display of the results was done using the tbss_fill command to ease visualization. The significant voxels were labeled according to the John Hopkins University white-matter tractography atlas available in FSL.58, 59 For more detailed information about data acquisition, processing and quantification of results see Supplementary III paragraph Imaging data acquisition and analysis. These data were only available in the hypothesis-confirming sample. Out of 707 subjects with complete data sets from the genetic association analysis, 342 subjects had complete DTI, mean arousal rating and genotype data.
DTI analysis—genotype-independent association analyses between DTI measures and mean positive arousal rating
The linear associations between mean positive arousal ratings and FA, as well as MD were tested, including age and sex as covariates. We also performed analyses of first eigenvalue (L1) and radial diffusivity.
DTI analysis—genotype-dependent differences in MD and FA
Further, we compared voxel-wise MD and FA values between genotype groups using a linear model, including age and sex as covariates.
DTI analysis—genotype-dependent differences in tracts with a significant association between MD and mean positive arousal rating
In a linear association analysis, we tested the significance of the interaction term between mean positive arousal and genotype using the averaged MD value per subject from all the voxels showing a significant association between mean positive arousal ratings and MD as dependent variable.
Results
Genetic association analysis
In the hypothesis-testing sample (N=1171), the genotype–phenotype association of four SNPs survived Westfall Young correction for multiple testing (number of tested SNPs: 85, number of phenotypes: 3). All SNPs showed a significant association with mean arousal ratings for positive pictures. The association between these SNPs and mean arousal ratings for negative or neutral pictures was not significant, neither at the corrected nor at the nominal significance level (for an overview see Supplementary Table S2). The association with mean arousal ratings for positive pictures was replicated for 1 of the 4 SNPs (rs2579372) in the hypothesis-confirming sample of 707 subjects (Table 1). The direction of effect and genetic model (recessive) were the same as in the hypothesis-testing sample (for an overview see Figure 1). Specifically, T-allele carriers of rs2579372 rated positive pictures as more arousing than non-T-allele carriers.
Association between 85 NTRK2 gene SNPs (recessive model) and mean positive arousal ratings (a) for the hypothesis-testing and -confirming sample separately as well as (b) in the combined sample. In a and b, the significant SNP rs2579372 is marked by a circle and its position is highlighted by a dotted vertical line. The white dots indicate the physical location of the 85 SNPs. The gene- and promoter-region are depicted by the peach and turquoise color, respectively. On the y-axis log-transformed P-values are represented and the x-axis depicts the chromosomal location in mega base pairs. The red dashed line represents the uncorrected P-value level <0.05. The lower panel shows the linkage disequilibrium (LD) structure of the NTRK2 locus in the hypothesis-testing sample. LD, linkage disequilibrium; SNP, single nucleotide polymorphism.
The additionally performed genome-wide analysis did not reveal any variant exceeding Bonferroni-corrected (P<5 × 10−8) significance.
Genotype-independent association between DTI measures and mean positive arousal rating
In 342 subjects of the hypothesis-confirming sample, we observed a significant whole-brain-corrected (PFWE<0.05) negative correlation between mean positive arousal ratings and MD (that is, higher MD correlated with lower mean arousal ratings for positive pictures) in several tracts of the right hemisphere, among others in the cingulum, inferior and superior longitudinal fasciculus (see Figure 2a and Table 2a). There was no significant (whole-brain corrected PFWE<0.05) positive correlation between MD and mean positive arousal ratings. Neither a negative nor a positive significant (whole-brain corrected PFWE<0.05) correlation between FA and mean positive arousal ratings was detected. We observed a whole-brain corrected (PFWE<0.05) negative correlation between mean positive arousal ratings and L1 (that is, higher L1 correlated with lower mean arousal ratings for positive pictures) bilaterally in the superior longitudinal fasciculus and several tracts of the right hemisphere including the anterior thalamic radiation, cingulum, corticospinal tract, inferior fronto-occipital and longitudinal fasciculus, and uncinate, as well as forceps minor and major (see Supplementary Table S3). No whole-brain corrected (PFWE<0.05) positive association between L1 and mean positive arousal ratings was observed. The radial diffusivity analysis did not reveal any significant (whole-brain corrected PFWE<0.05) positive or negative associations with mean positive arousal.
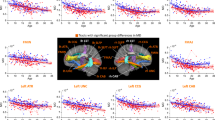
(a) Tracts with genotype-independent negative correlations between mean positive arousal ratings and MD (whole-brain corrected PFWE<0.05). (b) Tracts with significant (whole-brain corrected PFWE<0.05) genotype-dependent differences in MD. (c) Tracts with significant (whole-brain corrected PFWE<0.05) genotype-dependent differences in FA. FA, fractional anisotropy; L, left; MD, mean diffusivity; R, right.
Genotype-dependent differences in MD and FA
We identified a number of tracts with whole-brain-corrected genotype-dependent differences in MD, with T-allele carriers (n=276) of SNP rs2579372 having higher MD than non-T-allele carriers (n=66). These differences were mainly located bilaterally in white-matter fiber bundles connecting structures of the frontal and parietal lobes (see Figure 2b and Table 2b). There were no tracts showing significantly (whole-brain corrected PFWE<0.05) lower MD in T-allele carriers. In six out of the nine tracts showing a significant association between mean arousal ratings of positive pictures and MD, we observed as well significant differences in MD between genotype groups (see Figure 3 and Table 2 tracts marked with superscript c) on a tract- but not voxel-level.
Overlap of significant (whole-brain corrected PFWE<0.05) genotype-independent negative correlations between MD and mean positive arousal ratings (in blue color) and significant (whole-brain corrected PFWE<0.05) genotype-dependent MD differences (in red color) exemplarily in the forceps major and inferior fronto-occipital fasciculus (green circle). For a brain-wide overview see Supplementary Figure S1 in the supplementary. MD, mean diffusivity.
In addition, we observed a number of tracts with whole-brain corrected genotype-dependent differences in FA, with T-carriers (n=276) of SNP rs2579372 having lower FA than non-T-allele carriers (n=66). These differences were mainly located in the right hemisphere in white-matter fiber bundles connecting structures of the frontal and parietal lobes (see Figure 2c and Table 2c).
Genotype-dependent differences in tracts with a significant association between MD and mean positive arousal rating
Due to the overlap between significant tracts for the association of MD and mean arousal ratings of positive pictures, as well as genotype-dependent MD differences, we finally tested only in these overlapping voxels for a phenotype (mean positive arousal) × genotype interaction. There was no significant whole-brain-corrected (PFWE<0.05) interaction.
Discussion
We showed that a common SNP 5′ to NTRK2 (rs2579372) is associated with emotional arousal in two independent samples of healthy young subjects. T-allele carriers rated positive pictures as more arousing compared with non-T-allele carriers. In a genotype-independent analysis, we observed a negative correlation between mean positive arousal ratings and MD, a measure of brain white-matter integrity. We also observed rs2579372 genotype-dependent regional differences in MD and FA, with T-allele carriers having higher MD, respectively, lower FA values than non-T-allele carriers. Interestingly, the tracts showing genotype-dependent MD differences largely overlapped with the tracts showing significant correlations between MD and emotional arousal. However, the NTRK2 effects on emotional arousal and on MD were independent.
The genotype-dependent differences in emotional arousal ratings reported herein argue in favor of an involvement of NTRK2 in emotional processing in healthy subjects. To our knowledge this is the first report on the relation between NTRK2 and emotional processing in healthy subjects. So far several genetic associations between NTRK2 and psychopathology have been reported in the literature.31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 60, 61, 62 Given that dysregulation of emotional processes is a common characteristic of psychiatric disorders1 and that emotional arousal—specifically, the rating of emotionally arousing IAPS pictures—is reportedly altered in patients with psychiatric disorders,4, 5, 6, 7, 8, 9 we hypothesize that NTRK2 variants might be related to psychopathology through their influence on emotional processing. However, no study has hitherto investigated the direct link between NTRK2 and emotional dysregulation in psychopathology, and our results allow inferences solely in healthy populations. Nevertheless, the association between NTRK2 and emotional processing observed herein provides further ground for the investigation of the role of NTRK2 in emotional processes in patients with psychiatric disorders.
In our DTI analyses, we observed negative correlations between mean positive arousal ratings and a white-matter microstructure property (MD) in tracts that are mainly part of the limbic circuits (anterior thalamic radiation and cingulate gyrus) or form connections from other brain regions to these circuits (inferior fronto-occipital, inferior and superior longitudinal fasciculus).63 The limbic circuitry, along with its connections to other brain regions plays an essential role in emotion processing.29 The results of our DTI analysis are in line with this evidence. In addition, several studies report significant alterations in these tracts in patients with different psychiatric disorders like obsessive compulsive disorder, bipolar disorder, autism spectrum disorder, antisocial personality disorder and schizophrenia.64, 65, 66, 67, 68 Although the NTRK2 effects on emotional arousal and on MD were independent, it is of interest to note that the genotype-dependent differences in MD reported herein were regionally overlapping with the results of the correlation between arousal ratings and MD, and that engaged areas are involved in emotional processing29 and psychiatric disorders.64, 65, 66, 67, 68 In addition, we identified several tracts showing genotype-dependent differences in FA values (albeit in the absence of significant whole-brain-corrected correlations between FA and emotional arousal). Interestingly, these tracts overlapped substantially with the tracts showing genotype-dependent MD differences. Of note, Braskie et al.44, 45 reported significant NTRK1 and NTRK3 genotype-dependent differences in FA values in partially the same tracts, in which we identified NTRK2 genotype-dependent MD and FA differences. In our sample, the correlation between FA and MD values was highly significant (whole-brain average r(FA,MD)=−0.75, P=9.9e–128).
In addition to MD and FA, we also analyzed L1 and radial diffusivity values and observed significant correlations between L1 and mean positive arousal. L1 represents the largest eigenvalue of the fitted tensor or parallel diffusivity. We would like to stress that it is rather difficult to link this association to a specific axon-related characteristic (that is, axon diameter, axon count or axon density), given that the population under study consists uniquely of healthy young participants.69 Furthermore, because of the limitation of the tensor model in resolving regions of complex fiber architecture (for example, crossing fibers),70 the interpretation of DTI results to specific tracts is inherently limited. More advanced acquisition methods, such as high-angular-resolution diffusion imaging or diffusion spectrum imaging, might help disentangling this issue.71 These approaches are, however, usually more time-consuming and limit the investigation of large cohorts often needed in imaging genetics. Despite these methodological limitations, our results support an influence of neurotrophic receptor gene variants on white-matter microstructure.
In summary, our results suggest the involvement of a NTRK2 tag SNP in emotional processing and—independently—in brain white-matter integrity in healthy young subjects. Both the correlation of MD values with emotional arousal and the NTRK2 genotype-dependent differences in MD and FA values were located in brain regions known to be important for emotion processing in health and disease. These results may provide guidance for future studies on the role of NTRK2 in the mechanisms of emotion dysregulation.
References
Kring AM, Sloan DM . Emotion Regulation and Psychopathology: A Transdiagnostic Approach to Etiology and Treatment. The Guilford Press: New York, NY, USA, 2009.
Lang PJ, Öhmann A, Vaitl D The International Affective Picture System (slides). Center for Research in Psychophysiology, University of Florida: Gainesville, FL, USA. 1988.
Jayaro C, de la Vega I, Díaz-Marsá M, Montes A, Carrasco JL . The use of the International Affective Picture System for the study of affective dysregulation in mental disorders. Acta Esp Psiquiatr 2008; 36: 177–182.
Aguilar de Arcos F, Verdejo-García A, Ceverino A, Montañez-Pareja M, López-Juárez E, Sánchez-Barrera M et al. Dysregulation of emotional response in current and abstinent heroin users: negative heightening and positive blunting. Psychopharmacology (Berl) 2008; 198: 159–166.
Aguilar de Arcos F, Verdejo-García A, Peralta-Ramírez MI, Sánchez-Barrera M, Pérez-García M . Experience of emotions in substance abusers exposed to images containing neutral, positive, and negative affective stimuli. Drug Alcohol Depend 2005; 78: 159–167.
Aminoff S, Jensen J, Lagerberg T, Andreassen O, Melle I . Decreased self-reported arousal in schizophrenia during aversive picture viewing compared to bipolar disorder and healthy controls. Psychiatry Res 2011; 185: 309–314.
Jayaro C, De La Vega I, Bayon-Palomino C, Díaz-Marsá M, Montes A, Tajima K et al. Depressive-type emotional response pattern in impulsive-aggressive patients with borderline personality disorder. J Affect Disord 2011; 135: 37–42.
Strauss GP, Herbener ES . Patterns of emotional experience in schizophrenia: differences in emotional response to visual stimuli are associated with clinical presentation and functional outcome. Schizophr Res 2011; 128: 117–123.
Lee BT, Cho SW, Khang HS, Lee BC, Choi IG, Lyoo IK et al. The neural substrates of affective processing toward positive and negative affective pictures in patients with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 2007; 31: 1487–1492.
Bornovalova MA, Hicks BM, Iacono WG, McGue M . Longitudinal twin study of borderline personality disorder traits and substance use in adolescence: developmental change, reciprocal effects, and genetic and environmental influences. Personal Disord 2013; 4: 23–32.
Cardno AG, Gottesman II . Twin studies of schizophrenia: from bow-and-arrow concordances to star wars Mx and functional genomics. Am J Med Genet 2000; 97: 12–17.
Distel MA, Trull TJ, Derom CA, Thiery EW, Grimmer MA, Martin NG et al. Heritability of borderline personality disorder features is similar across three countries. Psychol Med 2008; 38: 1219–1229.
Goldman D, Oroszi G, Ducci F . The genetics of addictions: uncovering the genes. Nat Rev Genet 2005; 6: 521–532.
Sullivan PF, Neale MC, Kendler KS . Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry 2000; 157: 1552–1562.
Sullivan PF, Kendler KS, Neale MC . Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry 2003; 60: 1187–1192.
Duman RS, Heninger GR, Nestler EJ . A molecular and cellular theory of depression. Arch Gen Psychiatry 1997; 54: 597–606.
Duman RS, Monteggia LM . A neurotrophic model for stress-related mood disorders. Biol Psychiatry 2006; 59: 1116–1127.
Barbacid M . The Trk family of neurotrophin receptors. J Neurobiol 1994; 25: 1386–1403.
Klein R, Nanduri V, Jing SA, Lamballe F, Tapley P, Bryant S et al. The trkB tyrosine protein kinase is a receptor for brain-derived neurotrophic factor and neurotrophin-3. Cell 1991; 66: 395–403.
Klein R, Lamballe F, Bryant S, Barbacid M . The trkB tyrosine protein kinase is a receptor for neurotrophin-4. Neuron 1992; 8: 947–956.
Soppet D, Escandon E, Maragos J, Middlemas DS, Reid SW, Blair J et al. The neurotrophic factors brain-derived neurotrophic factor and neurotrophin-3 are ligands for the trkB tyrosine kinase receptor. Cell 1991; 65: 895–903.
Barbacid M . Structural and functional properties of the TRK family of neurotrophin receptors. Ann NY Acad Sci 1995; 766: 442–458.
Reichardt LF . Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci 2006; 361: 1545–1564.
Skaper SD . The biology of neurotrophins, signalling pathways, and functional peptide mimetics of neurotrophins and their receptors. CNS Neurol Disord Drug Targets 2008; 7: 46–62.
Klein R, Smeyne RJ, Wurst W, Long LK, Auerbach BA, Joyner AL et al. Targeted disruption of the trkB neurotrophin receptor gene results in nervous system lesions and neonatal death. Cell 1993; 75: 113–122.
Minichiello L . TrkB signalling pathways in LTP and learning. Nat Rev Neurosci 2009; 10: 850–860.
Colbran RJ . Thematic mini-review series: molecular mechanisms of synaptic plasticity. J Biol Chem 2015; 290: 28594–28595, jbc.R115.696468.
Musumeci G, Sciarretta C, Rodríguez-Moreno A, Al Banchaabouchi M, Negrete-Díaz V, Costanzi M et al. TrkB modulates fear learning and amygdalar synaptic plasticity by specific docking sites. J Neurosci 2009; 29: 10131–10143.
Dalgleish T . The emotional brain. Nat Rev Neurosci 2004; 5: 583–589.
Janak PH, Tye KM . From circuits to behaviour in the amygdala. Nature 2015; 517: 284–292.
Alonso P, Gratacòs M, Menchón JM, Saiz-Ruiz J, Segalàs C, Baca-García E et al. Extensive genotyping of the BDNF and NTRK2 genes define protective haplotypes against obsessive-compulsive disorder. Biol Psychiatry 2008; 63: 619–628.
Boulle F, Kenis G, Cazorla M, Hamon M, Steinbusch HWM, Lanfumey L et al. TrkB inhibition as a therapeutic target for CNS-related disorders. Prog Neurobiol 2012; 98: 197–206.
Ernst C, Wanner B, Brezo J, Vitaro F, Tremblay R, Turecki G . A deletion in tropomyosin-related kinase B and the development of human anxiety. Biol Psychiatry 2011; 69: 604–607.
Gupta VK, You Y, Gupta VB, Klistorner A, Graham SL . TrkB receptor signalling: implications in neurodegenerative, psychiatric and proliferative disorders. Int J Mol Sci 2013; 14: 10122–10142.
Hauger RL, Risbrough V, Oakley RH, Olivares-Reyes JA, Dautzenberg FM . Role of CRF receptor signaling in stress vulnerability, anxiety, and depression. Ann NY Acad Sci 2009; 1179: 120–143.
Hill RA . Interaction of sex steroid hormones and brain-derived neurotrophic factor-tyrosine kinase B signalling: relevance to schizophrenia and depression. J Neuroendocrinol 2012; 24: 1553–1561.
Kohli M, Salyakina D, Pfennig A, Lucae S, Horstmann S, Menke A et al. Association of genetic variants in the neurotrophic receptor-encoding gene NTRK2 and a lifetime history of suicide attempts in depressed patients. Arch Gen Psychiatry 2010; 67: 348–359.
Mahan AL, Ressler KJ . Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci 2012; 35: 24–35.
Marsden WN . Synaptic plasticity in depression: molecular, cellular and functional correlates. Prog Neuropsychopharmacol Biol Psychiatry 2013; 43: 168–184.
Deo AJ, Huang Y-Y, Hodgkinson CA, Xin Y, Oquendo MA, Dwork AJ et al. A large-scale candidate gene analysis of mood disorders: evidence of neurotrophic tyrosine kinase receptor and opioid receptor signaling dysfunction. Psychiatr Genet 2013; 23: 47–55.
Murphy ML, Carballedo A, Fagan AJ, Morris D, Fahey C, Meaney J et al. Neurotrophic tyrosine kinase polymorphism impacts white matter connections in patients with major depressive disorder. Biol Psychiatry 2012; 72: 663–670.
Klein R, Martin-Zanca D, Barbacid M, Parada LF . Expression of the tyrosine kinase receptor gene trkB is confined to the murine embryonic and adult nervous system. Development 1990; 109: 845–850.
Romanczyk TB, Weickert CS, Webster MJ, Herman MM, Akil M, Kleinman JE . Alterations in trkB mRNA in the human prefrontal cortex throughout the lifespan. Eur J Neurosci 2002; 15: 269–280.
Braskie MN, Kohannim O, Jahanshad N, Chiang M-C, Barysheva M, Toga AW et al. Relation between variants in the neurotrophin receptor gene, NTRK3, and white matter integrity in healthy young adults. Neuroimage 2013; 82: 146–153.
Braskie MN, Jahanshad N, Stein JL, Barysheva M, Johnson K, McMahon KL et al. Relationship of a variant in the NTRK1 gene to white matter microstructure in young adults. J Neurosci 2012; 32: 5964–5972.
Basser PJ . Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed 1995; 8: 333–344.
Beaulieu C . The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed 2002; 15: 435–455.
Sen PN, Basser PJ . A model for diffusion in white matter in the brain. Biophys J 2005; 89: 2927–2938.
Bellenguez C, Strange A, Freeman C, Donnelly P, Spencer CCA . A robust clustering algorithm for identifying problematic samples in genome-wide association studies. Bioinformatics 2012; 28: 134–135.
Westfall PH, Young SS . Resampling-Based Multiple Testing: Examples and Methods for P-Value Adjustment. John Wiley & Sons, Inc.: New York, NY, USA, 1993.
Gard MG, Kring AM . Sex differences in the time course of emotion. Emotion 2007; 7: 429–437.
Grühn D, Scheibe S . Age-related differences in valence and arousal ratings of pictures from the International Affective Picture System (IAPS): do ratings become more extreme with age? Behav Res Methods 2008; 40: 512–521.
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007; 81: 559–575.
Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 2006; 31: 1487–1505.
Nichols TE, Holmes AP . Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 2002; 15: 1–25.
Winkler AM, Ridgway GR . Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage 2014; 92: 381–397.
Smith SM, Nichols TE . Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 2009; 44: 83–98.
Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PCM, Mori S . Fiber tract-based atlas of human white matter anatomy. Radiology 2004; 230: 77–87.
Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage 2008; 39: 336–347.
Li Z, Zhang Y, Wang Z, Chen J, Fan J, Guan Y et al. The role of BDNF, NTRK2 gene and their interaction in development of treatment-resistant depression: data from multicenter, prospective, longitudinal clinic practice. J Psychiatr Res 2013; 47: 8–14.
Lin E, Hong C-J, Hwang J-P, Liou Y-J, Yang C-H, Cheng D et al. Gene-gene interactions of the brain-derived neurotrophic-factor and neurotrophic tyrosine kinase receptor 2 genes in geriatric depression. Rejuvenation Res 2009; 12: 387–393.
Ribases M, Gratacos M, Badia A, Jimenez L, Solano R, Vallejo J et al. Contribution of NTRK2 to the genetic susceptibility to anorexia nervosa, harm avoidance and minimum body mass index. Mol Psychiatry 2005; 10: 851–860.
Nieuwenhuys R, Voogd J, Huijzen C . The Human Central Nervous System: A Synopsis and Atlas. Springer-Verlag Berlin, Heidelberg, New York, NY, USA, 2008.
Sundram F, Deeley Q, Sarkar S, Daly E, Latham R, Craig M et al. White matter microstructural abnormalities in the frontal lobe of adults with antisocial personality disorder. Cortex 2012; 48: 216–229.
Fontenelle LF, Bramati IE, Moll J, Medlowicz M V, de Oliveira-Souza R, Tovar-Moll F . White matter changes in OCD revealed by diffusion tensor imaging. CNS Spectr 2011; 16: 101–109.
Lu LH, Zhou XJ, Keedy SK, Reilly JL, Sweeney JA . White matter microstructure in untreated first episode bipolar disorder with psychosis: comparison with schizophrenia. Bipolar Disord 2011; 13: 604–613.
Travers BG, Adluru N, Ennis C, Tromp DPM, Destiche D, Doran S et al. Diffusion tensor imaging in autism spectrum disorder: a review. Autism Res 2012; 5: 289–313.
Zanetti M V, Jackowski MP, Versace A, Almeida JRC, Hassel S, Duran FLS et al. State-dependent microstructural white matter changes in bipolar I depression. Eur Arch Psychiatry Clin Neurosci 2009; 259: 316–328.
Jones DK (ed). Diffusion MRI: Theory, Methods, and Applications. Oxford University Press, Oxford, UK, 2010.
Jbabdi S, Behrens TEJ, Smith SM . Crossing fibres in tract-based spatial statistics. Neuroimage 2010; 49: 249–256.
Abhinav K, Yeh F-C, Pathak S, Suski V, Lacomis D, Friedlander RM et al. Advanced diffusion MRI fiber tracking in neurosurgical and neurodegenerative disorders and neuroanatomical studies: A review. Biochim Biophys Acta - Mol Basis Dis 2014; 1842: 2286–2297.
Acknowledgements
KS conducted the study of the hypothesis-confirming sample, wrote the manuscript and analyzed the data. DC performed the imaging analyses and wrote the manuscript. VF performed quality control of genetic data and wrote the manuscript. FH conducted the study of the hypothesis-testing sample and wrote the manuscript. AH and AM provided technical assistance in genetic analyses and wrote the manuscript. DdQ and AP designed the study and wrote the manuscript. This work was funded by the Swiss National Science Foundation (Sinergia grants CRSI33 130080, CRSII1 136227 to DdQ and AP) and by the European Community‘s Seventh Framework Programme (FP7/2007–2013) under grant agreement # 602450 (IMAGEMEND).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Translational Psychiatry website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Spalek, K., Coynel, D., Freytag, V. et al. A common NTRK2 variant is associated with emotional arousal and brain white-matter integrity in healthy young subjects. Transl Psychiatry 6, e758 (2016). https://doi.org/10.1038/tp.2016.20
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/tp.2016.20
This article is cited by
-
Primate-specific stress-induced transcription factor POU2F1Z protects human neuronal cells from stress
Scientific Reports (2021)
-
A major role for common genetic variation in anxiety disorders
Molecular Psychiatry (2020)
-
Investigating the genetic profile of dopaminergic neurons in the VTA in response to perinatal nicotine exposure using mRNA-miRNA analyses
Scientific Reports (2018)