Abstract
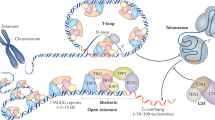
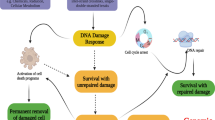
The molecular cloning of telomerase and telomere components has enabled the analysis and precise manipulation of processes that regulate telomere length maintenance. In mammalian cells and in other organisms, we now recognize that disruption of telomere integrity via any one of a number of perturbations induces chromosome instability and the activation of DNA damage responses. Thus, telomere dysfunction may represent a physiological trigger of the DNA damage or apoptotic response in an analogous fashion to other genotoxic insults that introduce chromosome breaks. Initial studies in mice lacking the murine telomerase RNA and in cells expressing a dominant negative version of the telomere binding protein TRF2 revealed a strong p53-dependent response to telomere dysfunction. Yet, telomere dysfunction exhibits p53-independent effects as well, an observation supported by p53-independent responses to telomere dysfunction in p53 mutant human tumor cell lines and mouse cells. As most tumors are compromised for p53 function, examination of this p53-independent response warrants closer attention. A better understanding of this p53-independent response may prove critical for determining the ultimate utility of telomerase inhibitors in the clinic. This review will summarize our current understanding of the molecular responses to telomere dysfunction in mammalian cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Artandi SE, Chang S, Lee SL, Alson S, Gottlieb GJ, Chin L, DePinho RA . 2000 Nature 406: 641–645
Beattie TL, Zhou W, Robinson MO, Harrington L . 1998 Curr. Biol. 8: 177–180
Blasco MA, Funk W, Villeponteau B, Greider CW . 1995 Science 269: 1267–1270
Blasco MA, Lee HW, Hande MP, Samper E, Landsorp PM, DePinho RA, Greider CW . 1997 Cell 91: 25–34
Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE . 1998 Science 279: 349–352
Broccoli D, Smogorzewska A, Chong L, de Lange T . 1997 Nature Genet. 17: 231–235
Bryan TM, Englezou A, Dalla-Pozza L, Dunham MA, Reddel RR . 1997 Nature Med. 3: 1271–1274
Chin L, Artandi SE, Shen Q, Tam A, Lee SL, Gottlieb GJ, Greider CW, DePinho RA . 1999 Cell 97: 527–538
Chong L, Van S-B, Broccoli D, Erdjument B-H, Hanish J, Tempst P, DeLange T . 1995 Science 270: 1663–1667
Colgin LM, Wilkinson C, Englezou A, Kilian A, Robinson MO, Reddel RR . 2000 Neoplasia 2: 426–432
Counter CM, Avillion AA, LeFeuvre CE, Stewart NG, Greider CW, Harley CB, Bacchetti S . 1992 EMBO J. 11: 1921–1929
de Lange T, Shiue L, Myers RM, Cox DR, Naylor SL, Killery AM, Varmus HE . 1990 Mol. Cell. Biol. 10: 518–527
De Laurenzi V, Melino G . 2000 Ann. NY Acad. Sci. 926: 90–100
Dunham MA, Neumann AA, Fasching CL, Reddel RR . 2000 Nature Genet. 26: 447–450
Engelhardt M, Drullinsky P, Guillem J, Moore MA . 1997 Clin. Cancer Res. 3: 1931–1941
Engelhardt M, Ozkaynak MF, Drullinsky P, Sandoval C, Tugal O, Jayabose S, Moore MAS . 1998 Leukemia 12: 13–24
Greider CW . 1996 Annu. Rev. Biochem. 65: 377–365
Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, de Lange T, Karlseder J, Broccoli D, Dai Y, Hardy S . 1999 Cell 97: 503–514
Guiducci C, Cerone MA, Bacchetti S . 2001 Oncogene 20: 714–725
Guo C, Geverd D, Liao R, Hamad N, Counter CM, Price DT . 2001 J. Urol. 166: 694–698
Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA . 1999a Nature 400: 464–468
Hahn WC, Stewart SA, Brooks MW, York SG, Eaton E, Kurachi A, Beijersbergen RL, Knoll JH, Meyerson M, Weinberg RA . 1999b Nature Med. 5: 1164–1170
Hakem R, de la Pompa JL, Elia A, Potter J, Mak TW . 1997 Nature Genet. 16: 298–302
Harley CB . 1991 Mutat. Res. 256: 271–282
Harley CB . 1996 Telomeres Greider EHBaCW (ed) Cold Spring Harbor: Cold Spring Harbor Laboratory Press pp 247–263
Harrington L, Zhou W, McPhail T, Oulton R, Yeung DS, Mar V, Bass MB, Robinson MO . 1997 Genes Dev. 11: 3109–3115
Hastie ND, Dempster M, Dunlop MG, Thompson AM, Green DK, Allshire RC . 1990 Nature 346: 866–868
Herbert BS, Pitts AE, Baker SI, Hamilton SE, Wright WE, Shay JW, Corey DR . 1999 Proc. Natl. Acad. Sci. USA 96: 14276–14281
Karlseder J, Broccoli D, Dai Y, Hardy S, de Lange T . 1999 Science 283: 1321–1325
Kass-Eisler A, Greider CW . 2000 Trends Biochem. Sci. 25: 200–204
Kim MM, Rivera MA, Botchkina IL, Shalaby R, Thor AD, Blackburn EH . 2001 Proc. Natl. Acad. Sci. USA 98: 7982–7987
Lee HW, Blasco MA, Gottlieb GJ, Horner JW, Greider CW, DePinho RA . 1998 Nature 392: 569–574
Lee KH, Rudolph KL, Ju Y, Greenberg RA, Cannizzaro L, Chin L, Weiler SR, DePinho RA . 2001 Proc. Natl. Acad. Sci. USA 98: 3381–3386
Lingner J, Hughes TR, Shevchenko A, Mann M, Lundblad V, Cech TR . 1997 Science 276: 561–567
Ludwig A, Saretzki G, Holm PS, Tiemann F, Lorenz M, Emrich T, Harley CB, von Zglinicki T . 2001 Cancer Res. 61: 3053–3061
Marusic L, Anton M, Tidy A, Wang P, Villeponteau B, Bacchetti S . 1997 Mol. Cell. Biol. 17: 6394–6401
Morales CP, Holt SE, Ouellette M, Kaur KJ, Yan Y, Wilson KS, White MA, Wright WE, Shay JW . 1999 Nature Genet. 21: 115–118
Nakayama J, Tahara H, Tahara E, Saito M, Ito K, Nakamura H, Nakanishi T, Tahara E, Ide T, Ishikawa F . 1998 Nature Genet. 18: 65–68
Rudolph KL, Chang S, Lee HW, Blasco M, Gottlieb GJ, Greider C, DePinho RA . 1999 Cell 96: 701–712
Rudolph KL, Millard M, Bosenberg MW, DePinho RA . 2001 Nature Genet. 28: 155–159
Shay JW, Bacchetti S . 1997 Eur. J. Cancer 33: 787–791
van Steensel B, Smorgorzewska A, De Lange T . 1998 Cell 92: 401–413
Vaziri H, Benchimol S . 1998 Curr. Biol. 8: 279–282
Vaziri H, Squire JA, Pandita TK, Bradley G, Kuba RM, Zhang H, Gulyas S, Hill RP, Nolan GP, Benchimol S . 1999 Mol. Cell. Biol. 19: 2373–2379
Weinrich SL, Pruzan R, Ma L, Ouellette M, Tesmer VM, Holt SE, Bodnar AG, Lichtsteiner S, Kim NW, Trager JB, Taylor RD, Carlos R, Andrews WH, Wright WE, Shay JW, Harley CB, Morin GB . 1997 Nature Genet. 17: 498–502
Wong KK, Chang S, Weiler SR, Ganesan S, Chaudhuri J, Zhu C, Artandi SE, Rudolph KL, Gottlieb GJ, Chin L, Alt FW, DePinho RA . 2000 Nature Genet 26: 85–88
Yi X, White DM, Aisner DL, Baur JA, Wright WE, Shay JW . 2000 Neoplasia 2: 433–440
Zhang X, Mar V, Zhou W, Harrington L, Robinson MO . 1999 Genes Dev. 13: 2388–2399
Zhu XD, Kuster B, Mann M, Petrini JH, de Lange T . 2000 Nature Genet. 25: 347–352
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harrington, L., Robinson, M. Telomere dysfunction: multiple paths to the same end. Oncogene 21, 592–597 (2002). https://doi.org/10.1038/sj.onc.1205084
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.onc.1205084
Keywords
This article is cited by
-
Estrogen deficiency reversibly induces telomere shortening in mouse granulosa cells and ovarian aging in vivo
Protein & Cell (2011)
-
Senescence and life span
Pflügers Archiv - European Journal of Physiology (2010)
-
TRF2 functions as a protein hub and regulates telomere maintenance by recognizing specific peptide motifs
Nature Structural & Molecular Biology (2009)
-
Telomere-Mediated Effects on Melanogenesis and Skin Aging
Journal of Investigative Dermatology Symposium Proceedings (2009)
-
Does the reservoir for self-renewal stem from the ends?
Oncogene (2004)