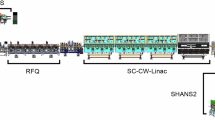
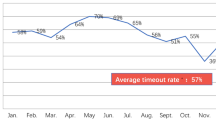
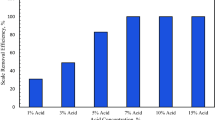
Abstract
It has been shown by numerous investigators that potassium and salts of potassium are radioactive, the activity being due to the emission of β-particles by the atoms of potassium. In accordance with the radioactive displacement law, Hahn and Rothenbach (Phys. Zeit., 20, p. 194; 1919) pointed out that this β-transformation of potassium should result in the production of an isotope of calcium, without change in atomic weight. On the other hand, S. Rosseland (Zeit. f. Phys., 14, p. 173; 1923) has put forward an ingenious suggestion which does not necessitate the production of a calcium isotope. Later, Hevesy and Løgstrup (Zeit. anorg. Chem., 171, p. 1; 1928) showed experimentally that the radioactivity of potassium appears to be confined to the isotope of mass 41; Aston had previously shown that potassium consists of two isotopes of masses 39 and 41. If only the isotope of mass 41 is radioactive, Holmes and Lawson (Phil. Mag., 2, p. 1218; 1926) worked out the half-value period of this isotope, and on the basis of this result, Hevesy and Løgstrup calculated that, if we assume the earth to have existed for 109 years, about 0.001 of all the potassium in the earth must have been transformed into calcium of atomic weight 41.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
FROST, A., FROST, O. The Product of the Radioactive Disintegration of Potassium. Nature 125, 48 (1930). https://doi.org/10.1038/125048a0
Issue date:
DOI: https://doi.org/10.1038/125048a0