Abstract
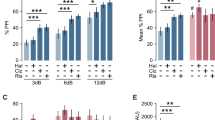
Prepulse inhibition (PPI) refers to the reduction in startle reaction to a startle-eliciting stimulus when it is shortly preceded by a subthreshold prepulse stimulus. PPI has been extensively employed as an assay for sensorimotor gating, and its disruption has been characterized in specific disease conditions, including schizophrenia. In animals, dopamine agonists disrupt PPI, and this disruption can be antagonized by antipsychotic drug treatment. The present study extended these fundamental findings to C57BL6 mice, and further evaluated the subjects' reaction to the prepulse stimulus alone in relation to the expression of PPI. Not only did apomorphine (2.0 mg/kg, intraperitoneal (i.p.)) attenuate PPI but it also enhanced reactivity to the prepulse stimulus. The dual effects of apomorphine appear paradoxical in view of the positive correlation, detectable in both the control and apomorphine groups, between prepulse reactivity and PPI magnitude. The present findings contradict the hypothesis that apomorphine disrupts PPI via reduced detectability or perception of the prepulse, and we further propose that enhanced distractibility may provide a parsimonious account for the dual effects of apomorphine. Moreover, haloperidol pretreatment (0.4 mg/kg, i.p.) fully antagonized the effects of apomorphine upon prepulse reactivity as well as on PPI. The present results add to our understanding of the relevance and applicability of the PPI paradigm in modeling schizophrenia-like symptoms in animals.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Notes
We conducted additional runs of the present PPI protocol with four dead mice, and confirmed that none of these sessions generated consistent readings on prepulse-alone trials as seen in the three experiments reported here. This indicated that our measurements of prepulse reactivity did not stem from spurious vibrations associated with the delivery of the prepulse stimulus itself.
References
Braff DL, Geyer MA, Swerdlow NR (2001). Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology 156: 234–258.
Braff DL, Grillon C, Geyer MA (1992). Gating and habituation of startle reflex in schizophrenic patients. Arch Gen Psychiatry 49: 206–215.
Castellanos FX, Fine EJ, Kaysen D, Marsh WL, Rapoport JL, Hallett M (1996). Sensorimotor gating in boys with Tourette's syndrome and ADHD: preliminary results. Biol Psychiatry 39: 33–41.
Chen PY, Popovich PM (2002). Correlation: Parametric and Nonparametric Measures. Sage Publications, Inc.: Newbury Park, CA.
Davis M, Mansbach RS, Swerdlow NR, Campeau S, Braff DL, Geyer MA (1990). Apomorphine disrupts the inhibition of acoustic startle induced by weak prepulses in rats. Psychopharmacology 102: 1–4.
Filion DL, Dawson ME, Schell AM (1993). Modification of the acoustic startle-reflex eyeblink: a tool for investigating early and late attentional processes. Biol Psychol 35: 185–200.
Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR (2001). Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology 156: 117–154.
Geyer MA, McIlwain KL, Paylor R (2002). Mouse genetic models for prepulse inhibition: an early review. Mol Psychiatry 7: 1039–1053.
Graham FK (1975). The more or less startling effects of weak prestimulation. Psychophysiology 12: 238–248.
Graham FK (1980). Control of blink reflex excitability. In: Thompson RF, Hicks LH, Shvyrkov VB (eds). Neural Mechanisms of Goal-Directed Behavior and Learning. Academic Press: New York. pp 511–519.
Graham FK (1992). Attention: the heartbeat, the blink, and the brain. In: Campbell BA, Hayne H, Richardson R (eds). Attention and Information Processing in Infants and Adults: Perspectives from Human and Animal Research. Lawrence Erlbaum Associates: Hillsdale, NJ. pp 3–29.
Hoffman HS, Searle JR (1965). Acoustic variables in the modification of startle reaction in the rat. J Comp Exp Psychol 60: 53–58.
Kraeplin E (1919). Dementia Praecox and Paraphrenia (Translated by R.M. Barclay) Robert E. Krieger (1971): Huntington, NY.
Norris CM, Blumenthal TD (1996). A relationship between inhibition of the acoustic startle response and the protection of prepulse processing. Psychobiology 24: 160–168.
Nuechterlein KH, Dawson ME (1984). Information processing and attentional functioning in the developmental course of schizophrenic disorders. Schizophr Bull 10: 160–203.
Postma P, Kumari V, Hines M, Gray JA (2001). The relationship between prepulse detection and prepulse inhibition of the acoustic startle reflex. Psychophysiology 38: 377–382.
Servan-Schreiber D, Printz H, Cohen JD (1990). A network model of catecholamine effects: gain, signal-to-noise ratio, and behavior. Science 249: 892–895.
Snyder SH (1976). The dopamine hypothesis of schizophrenia: focus on the dopamine receptor. Am J Psychiatry 133: 197–202.
Swerdlow NR, Braff DL, Geyer MA (2000). Animal models of deficient sensorimotor gating: what we know, what we think we know, and what we hope to know soon. Behav Pharmacol 11: 185–204.
Swerdlow NR, Braff DL, Taaid N, Geyer MA (1994). Assessing the validity of an animal model of deficient sensorimotor gating in schizophrenic patients. Arch Gen Psychiatry 51: 139–154.
Swerdlow NR, Geyer MA (1998). Using an animal model of deficient sensorimotor gating to study the pathophysiology and new treatments of schizophrenia. Schizophr Bull 24: 285–301.
Swerdlow NR, Paulsen J, Braff DL, Butters N, Geyer MA, Swenson MR (1995). Impaired prepulse inhibition of acoustic and tactile startle response in patients with Huntington's disease. J Neurol Neurosurg Psychiatry 58: 192–200.
Winer BJ (1971). Statistical Principles in Experimental Design. McGraw-Hill Inc.: New York.
Yee BK (2000). Cytotoxic lesion of the medial prefrontal cortex abolishes the partial reinforcement extinction effect, attenuates prepulse inhibition of the acoustic startle reflex and induces transient hyperlocomotion, while sparing spontaneous object recognition memory in the rat. Neuroscience 95: 675–689.
Zorrilla EP (1997). Multiparous species present problems (and possibilities) to developmentalists. Dev Psychobiol 30: 141–150.
Acknowledgements
This study was supported by the Swiss Federal Institute of Technology, Zurich. BK Yee was further supported by the National Center of Competence in Research (NCCR): Neural Plasticity and Repair, Swiss National Science Foundation. We gratefully acknowledge Dr Tobias Bast's efforts in critical thinking, and thank Alan Ipekian and Peter Schmid for their technical and editorial expertise. We also remain indebted to the anonymous reviewers for their constructive criticism in the revision of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yee, B., Russig, H. & Feldon, J. Apomorphine-Induced Prepulse Inhibition Disruption is Associated with a Paradoxical Enhancement of Prepulse Stimulus Reactivity. Neuropsychopharmacol 29, 240–248 (2004). https://doi.org/10.1038/sj.npp.1300323
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.npp.1300323
Keywords
This article is cited by
-
Dissociable effects of the d- and l- enantiomers of govadine on the disruption of prepulse inhibition by MK-801 and apomorphine in male Long-Evans rats
Psychopharmacology (2017)
-
Baseline prepulse inhibition expression predicts the propensity of developing sensitization to the motor stimulant effects of amphetamine in C57BL/6 mice
Psychopharmacology (2013)
-
SSR504734 enhances basal expression of prepulse inhibition but exacerbates the disruption of prepulse inhibition by apomorphine
Psychopharmacology (2013)
-
A conceptual and practical guide to the behavioural evaluation of animal models of the symptomatology and therapy of schizophrenia
Cell and Tissue Research (2013)
-
Prepulse inhibition of auditory change-related cortical responses
BMC Neuroscience (2012)