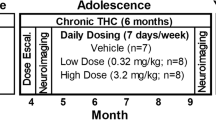
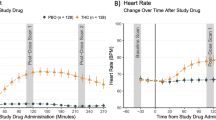
Abstract
Despite concerns surrounding the possible adverse effects of marijuana on complex cognitive function, the processes contributing to the observed cognitive deficits are unclear, as are the causal relationships between these impairments and marijuana exposure. In particular, marijuana-related deficits in cognitive flexibility may affect the social functioning of the individual and may contribute to continued marijuana use. We therefore examined the ability of rats to perform affective and attentional shifts following acute administration of Δ9-tetrahydrocannabinol (THC), the primary psychoactive marijuana constituent. Administration of 1 mg/kg THC produced marked impairments in the ability to reverse previously relevant associations between stimulus features and reward presentation, while the ability to transfer attentional set between dimensional stimulus properties was unaffected. Concurrent in situ hybridization analysis of regional c-fos and ngfi-b expression highlighted areas of the prefrontal cortex and striatum that were recruited in response to both THC administration and task performance. Furthermore, the alterations in mRNA expression in the orbitofrontal cortex and striatum were associated with the ability to perform the reversal discriminations. These findings suggest that marijuana use may produce inelasticity in updating affective associations between stimuli and reinforcement value, and that this effect may arise through dysregulation of orbitofrontal and striatal circuitry.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Atha M (2003). Cannabis Use in Britain—Independent Drug Monitoring Unit. www.idmu.co.uk/canuseuk.htm. Accessed 1 February 2005.
Barense MD, Fox MT, Baxter MG (2002). Aged rats are impaired on an attentional set-shifting task sensitive to medial frontal cortex damage in young rats. Learn Memory 9: 191–201.
Berke JD, Paletzki RF, Aronson GJ, Hyman SE, Gerfen CR (1998). A complex program of striatal gene expression induced by dopaminergic stimulation. J Neurosci 18: 5301–5310.
Berridge KC, Robinson TE (1998). What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev 28: 309–369.
Birrell JM, Brown VJ (2000). Medial prefrontal cortex mediates perceptual attention set shifting in the rat. J Neurosci 20: 4320–4324.
Block RI (1996). Does heavy marijuana use impair human cognition and brain function? JAMA 275: 560–561.
Bloom AS, Tershner S, Fuller SA, Stein EA (1997). Cannabinoid-induced alterations in regional cerebral blood flow in the rat. Pharmacol Biochem Behav 57: 625–631.
Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL (2002). Dose-related neurocognitive effects of marijuana use. Neurology 59: 1337–1343.
Butter C (1969). Perseveration in extinction and in discrimination reversal tasks following selective frontal ablations in Macaca mulatta. Physiol Behav 4: 163–171.
Cardinal RN, Parkinson JA, Hall J, Everitt BJ (2002). Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev 26: 321–352.
Chait LD, Perry JL (1994). Acute and residual effects of alcohol and marijuana, alone and in combination, on mood and performance. Psychopharmacology (Berl) 115: 340–349.
Chudasama Y, Bussey TJ, Muir JL (2001). Effects of selective thalamic and prelimbic cortex lesions on two types of visual discrimination and reversal learning. Eur J Neurosci 14: 1009–1020.
Cools R, Clark L, Owen AM, Robbins TW (2002). Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. J Neurosci 22: 4563–4567.
Cools R, Clark L, Robbins TW (2004). Differential responses in human striatum and prefrontal cortex to changes in object and rule relevance. J Neurosci 24: 1129–1135.
Crofts HS, Dalley JW, Collins P, Van Denderen JCM, Everitt BJ, Robbins TW et al (2001). Differential effects of 6-OHDA lesions of the frontal cortex and caudate nucleus on the ability to acquire and attentional set. Cereb Cortex 11: 1015–1026.
Curran T, Gordon MB, Rubino KL, Sambucetti LC (1987). Isolation and characterization of the c-fos(rat) cDNA and analysis of post-translational modification in vitro. Oncogene 2: 79–84.
Devane WA, Dysarz FA, Johnson MR, Melvin LS, Howlett AC (1988). Determination and characterisation of a cannabinoid receptor in the rat brain. Mol Pharmacol 34: 604–613.
Dias R, Robbins TW, Roberts AC (1996a). Dissociation in prefrontal cortex of affective and attentional shifts. Nature 380: 69–72.
Dias R, Robbins TW, Roberts AC (1996b). Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci 110: 872–886.
Dias R, Robbins TW, Roberts AC (1997). Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin Card Sort Test: restriction to novel situations and independence from ‘on-line’ processing. J Neurosci 17: 9285–9297.
Divac I, Rosvold HE, Szwarcbart MK (1967). Behavioral effects of selective ablation of the caudate nucleus. J Comp Physiol Psychol 63: 184–190.
Eimas PD (1966). Effects of overtraining and age on intradimensional and extradimensional shifts in children. J Exp Child Psychol 3: 348–355.
Erdtmann-Vourliotis M, Mayer P, Riechert U, Hollt V (1999). Acute injection of drugs with low addictive potential (Δ9-tetrahydrocannabinol, 3,4-methylenedioxymethamphetamine, lysergic acid diamide) causes a much higher c-fos expression in limbic brain areas than highly addicting drugs (cocaine and morphine). Mol Brain Res 71: 313–324.
Fant RV, Heishman SJ, Bunker EB, Pickworth WB (1998). Acute and residual effects of marijuana in humans. Pharmacol Biochem Behav 60: 777–784.
Ferry AT, Lu XC, Price JL (2000). Effects of excitotoxic lesions in the ventral striatopallidal–thalamocortical pathway on odor reversal learning: inability to extinguish an incorrect response. Exp Brain Res 131: 320–335.
Fletcher JM, Page B, Francis DJ, Copeland K, Naus MJ, Davis CM et al (1996). Cognitive correlates of long-term cannabis use in Costa Rican men. Arch Gen Psychiatry 53: 1051–1057.
Fox MT, Barense MD, Baxter MG (2003). Perceptual attentional set-shifting is impaired in rats with neurotoxic lesions of posterior parietal cortex. J Neurosci 23: 676–681.
Glass M, Dragunow M, Faull RL (1997). Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience 77: 299–318.
Hart CL, van Gorp W, Haney M, Foltin RW, Fischman MW (2001). Effects of acute smoked marijuana on complex cognitive performance. Neuropsychopharmacology 25: 757–765.
Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC (1991a). Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci 11: 563–583.
Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC (1991b). Characterisation and localisation of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci 11: 563–583.
Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR et al (1990). Cannabiniod receptor localisation in brain. Proc Natl Acad Sci USA 87: 1932–1936.
Higgs S, Williams CM, Kirkham TC (2003). Cannabinoid influences on palatability: microstructural analysis of sucrose drinking after delta(9)-tetrahydrocannabinol, anandamide, 2-arachidonoyl glycerol and SR141716. Psychopharmacology (Berl) 165: 370–377.
Iversen SD, Mishkin M (1970). Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Exp Brain Res 11: 376–386.
Jentsch JD, Taylor JR (1999). Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 146: 373–390.
Jones B, Mishkin M (1972). Limbic lesions and the problem of stimulus–reinforcement associations. Exp Neurol 36: 362–377.
Liraud F, Verdoux H (2000). Which temperamental characteristics are associated with substance use in subjects with psychotic and mood disorders? Psychiatry Res 93: 63–72.
Lundqvist T, Jonsson S, Warkentin S (2001). Frontal lobe dysfunction in long-term cannabis users. Neurotoxicol Teratol 23: 437–443.
Mailleux P, Verslype M, Preud'homme X, Vanderhaeghen J (1994). Activation of multiple transcription factor genes by tetrahydrocannabinol in rat forebrain. Neuroreport 5: 1265–1268.
Margulies JE, Hammer JRP (1991). Tetrahydrocannabinol alters cerebral metabolism in a biphasic, dose-dependent manner in rat brain. J Pharmacol 202: 373–378.
Mathew RJ, Wilson WH (1993). Acute changes in cerebral blood flow after smoking marijuana. Life Sci 52: 757–767.
Mathew RJ, Wilson WH, Coleman RE, Turkington TG, DeGrado TR (1997). Marijuana intoxication and brain activation in marijuana smokers. Life Sci 60: 2075–2089.
Mathew RJ, Wilson WH, Turkington TG, Hawk TC, Coleman RE, DeGrado TR et al (2002). Time course of tetrahydrocannabinol-induced changes in regional cerebral blood flow measured with positron emission tomography. Psychiatry Res 116: 173–185.
McAlonan K, Brown VJ (2003). Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res 146: 97–103.
McGregor IS, Arnold JC, Weber MF, Topple AN, Hunt GE (1998). A comparison of Δ9-THC and anandamide induced c-fos expression in the rat forebrain. Brain Res 802: 19–26.
Mechoulam R, Shani A, Edery H, Gruadfield Y (1970). Chemical basis of hashish activity. Science 169: 611–612.
Monchi O, Petrides M, Petre V, Worsley K, Dagher A (2001). Wisconsin card sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. J Neurosci 21: 7733–7741.
Mordenti J, Chappell W (1989). The use of interspecies scaling in toxicokinetics. In: Yacobi A, Kelly J, Batra V (eds). Toxicokinetics and New Drug Development. Pergamon Press: New York. pp 42–96.
Morgan JI, Curran T (1989). Stimulus-transcription coupling: immediate-early genes. Trends Neurosci 12: 459–462.
O'Leary DS, Block RI, Flaum M, Schultz SK, Boles Ponto LL, Watkins GL et al (2000). Acute marijuana effects on rCBF and cognition: a PET study. Neuroreport 11: 3835–3841.
O'Leary DS, Block RI, Koeppel JA, Flaum M, Schultz SK, Andreasen NC et al (2002). Effects of smoking marijuana on brain perfusion and cognition. Neuropsychopharmacology 26: 802–816.
Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW (1991). Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia 29: 993–1006.
Paxinos G, Watson C (1998). The Rat Brain in Stereotaxic Coordinates 4th edn. Academic Press: Sydney.
Persico AM, Uhl GR (1996). Transcription factors: potential roles in drug-induced neuroplasticity. Rev Neurosci 7: 233–275.
Pertwee RG, Stevenson LA, Elrick DB, Mechoulam R, Corbett AD (1992). Inhibitory effects of certain enantiomeric cannabinoids in the mouse vas deferens and the myenteric plexus preparation of guinea-pig small intestine. Br J Pharmacol 105: 980–984.
Pickworth WB, Rohrer MS, Fant RV (1997). Effects of abused drugs on psychomotor performance. Exp Clin Psychopharmacol 5: 235–241.
Pope HG, Gruber AJ, Hudson JI, Heustis MA, Yurgulen-Todd D (2001). Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry 58: 909–915.
Pope HG, Yurgelun-Todd D (1996). The residual cognitive effects of heavy marijuana use in college students. JAMA 275: 521–527.
Pope Jr HG (2002). Cannabis, cognition, and residual confounding. JAMA 287: 1172–1174.
Rogers RD, Andrews TC, Grasby PM, Brooks DJ, Robbins TW (2000). Contrasting cortical and subcortical activations produced by attentional-set shifting and reversal learning in humans. J Cogn Neurosci 12: 142–162.
Rolls ET (1996). The orbitofrontal cortex. Philos Trans R Soc London B 351: 1433–1443; discussion 1443–1444.
Rolls ET (2000). The orbitofrontal cortex and reward. Cereb Cortex 10: 284–294.
Rolls ET (2004). The functions of the orbitofrontal cortex. Brain Cogn 55: 11–29.
Sañudo-Peña MC, Romero J, Seale GE, Fernandez-Ruiz JJ, Walker JM (2000). Activational role of cannabinoids on movement. Eur J Pharm 391: 269–274.
Scheier LM, Botvin GJ (1996). Cognitive effects of marijuana. JAMA 275: 1547.
Schoenbaum G, Chiba AA, Gallagher M (2000). Changes in functional connectivity in orbitofrontal cortex and basolateral amygdala during learning and reversal training. J Neurosci 20: 5179–5189.
Schoenbaum G, Nugent SL, Saddoris MP, Setlow B (2002). Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport 13: 885–890.
Schoenbaum G, Setlow B (2003). Lesions of nucleus accumbens disrupt learning about aversive outcomes. J Neurosci 23: 9833–9841.
Solowij N, Stephens R, Roffman RA, Babor T (2002). Does marijuana use cause long-term cognitive deficits? JAMA 287: 2653–2654 ; author reply 2654.
Spinella M (2003). Relationship between drug use and prefrontal-associated traits. Addict Biol 8: 67–74.
Stern CE, Passingham RE (1995). The nucleus accumbens in monkeys (Macaca fascicularis). III. Reversal learning. Exp Brain Res 106: 239–247.
Tremblay L, Schultz W (1999). Relative reward preference in primate orbitofrontal cortex. Nature 398: 704–708.
Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ (2004). Catechol-o-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci 24: 5331–5335.
Volkow ND, Fowler JS (2000). Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex 10: 318–325.
Volkow ND, Gillespie H, Mullani N, Tancredi L, Grant C, Valentine A et al (1996). Brain glucose metabolism in chronic marijuana users at baseline and during marijuana intoxication. Psychiatry Res Neuroimag 67: 29–38.
Whitlow CT, Freedland CS, Porrino LJ (2002). Metabolic mapping of the time-dependent effects of delta 9-tetrahydrocannabinol administration in the rat. Psychopharmacology (Berl) 161: 129–136.
Winstanley CA, Theobald DE, Cardinal RN, Robbins TW (2004). Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci 24: 4718–4722.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Egerton, A., Brett, R. & Pratt, J. Acute Δ9-Tetrahydrocannabinol-Induced Deficits in Reversal Learning: Neural Correlates of Affective Inflexibility. Neuropsychopharmacol 30, 1895–1905 (2005). https://doi.org/10.1038/sj.npp.1300715
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.npp.1300715
Keywords
This article is cited by
-
Δ-9-Tetrahydrocannabinol and Cannabidiol produce dissociable effects on prefrontal cortical executive function and regulation of affective behaviors
Neuropsychopharmacology (2019)
-
Acute effects of cocaine and cannabis on reversal learning as a function of COMT and DRD2 genotype
Psychopharmacology (2016)
-
Activation of cannabinoid system in anterior cingulate cortex and orbitofrontal cortex modulates cost-benefit decision making
Psychopharmacology (2015)
-
Involvement of the endocannabinoid system in reward processing in the human brain
Psychopharmacology (2012)
-
Are adolescents more vulnerable to drug addiction than adults? Evidence from animal models
Psychopharmacology (2009)