Abstract
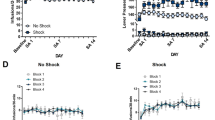
Cocaine addiction in humans is characterized by cycles of abstinence from drug-taking and relapse. Here, electrophysiological recording procedures were used to determine whether nucleus accumbens (Acb) neuronal firing properties are altered following interruption and resumption of cocaine self-administration. Rats (n=12) were trained to self-administer cocaine (2 h daily sessions) then divided into two groups. Acb activity was recorded for Group 1 (controls) during two additional self-administration sessions completed over the next 2 days (test sessions 1 and 2). Acb activity was recorded for Group 2 (1-month) during one self-administration session completed the next day (test 1), and during a second self-administration session 1 month later (test 2). As in prior reports, a subset of Acb neurons exhibited patterned discharges (short duration and/or long-term cyclic alterations, termed ‘phasically active’) relative to cocaine-reinforced responding during test session 1. Remarkably, the percentage of phasically active cells dramatically increased (nearly two-fold) following 1-month abstinence, in the core but not the shell of the Acb. Likewise, the strength of the neural correlates (determined via signal-to-baseline ratios) also increased as a function of abstinence. Extinction experiments in another set of rats (n=12) revealed an increased motivational state for the drug following abstinence. The results show that abstinence from cocaine self-administration causes a dramatic increase in the number and strength of Acb neurons that encode cocaine-related information, thus representing the first neurophysiological correlate of heightened activation of the ‘brain reward system’ following abstinence and resumption (relapse) of cocaine consumption.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ahmed SH, Koob GF (2005). Transition to drug addiction: a negative reinforcement model based on an allostatic decrease in reward function. Psychopharmacology 25 [Epub ahead of print].
Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S et al (2003). Neuroadaptations in the cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci 6: 743–749.
Caine SB, Lintz R, Koob GF (1993). In: Sahgal A (ed) Behavioral Neuroscience: A Practical Approach. Oxford UP: Oxford. pp 117–143.
Carelli RM (2000). Activation of accumbens cell firing by stimuli associated with cocaine delivery during self-administration. Synapse 35: 238–242.
Carelli RM (2002). Nucleus accumbens cell firing during goal-directed behaviors for cocaine vs ‘natural’ reinforcement. Physiol Behav 76: 379–387.
Carelli RM, Deadwyler SA (1994). A comparison of nucleus accumbens neuronal firing patterns during cocaine self-administration and water reinforcement in rats. J Neurosci 14: 7735–7746.
Carelli RM, Ijames S (2000). Nucleus accumbens cell firing during maintenance, extinction, and reinstatement of cocaine self-administration behavior in rats. Brain Res 866: 44–54.
Carelli RM, Ijames S, Konstantopoulos J, Deadwyler SA (1999). Examination of factors mediating the transition to behaviorally correlated nucleus accumbens cell firing during cocaine self-administration sessions in rats. Behav Brain Res 104: 127–139.
Carelli RM, Ijames SG (2001). Selective activation of accumbens neurons by cocaine-associated stimuli during a water/cocaine multiple schedule. Brain Res 907: 156–161.
Carelli RM, Ijames SG, Crumling AJ (2000). Evidence that separate circuits in the nucleus accumbens encode cocaine vs ‘natural’ (water and food) reward. J Neurosci 20: 4255–4266.
Carelli RM, King VC, Hampson RE, Deadwyler SA (1993). Firing patterns of nucleus accumbens neurons during cocaine self-administration in rats. Brain Res 626: 14–22.
Carelli RM, Wightman RM (2004). Functional microcircuitry in the accumbens underlying drug addiction: insights from real-time signaling during behavior. Curr Opin Neurobiol 14: 763–768.
Chang JY, Sawyer SF, Lee RS, Woodward DJ (1994). Electrophysiological and pharmacological evidence for the role of the nucleus accumbens in cocaine self-administration in freely moving rats. J Neurosci 14: 1224–1244.
Di Ciano P, Everitt BJ (2002). Reinstatement and spontaneous recovery of cocaine-seeking following extinction and different durations of withdrawal. Behav Pharmacol 13: 397–405.
Everitt BJ, Wolf ME (2002). Psychomotor stimulant addiction: a neural systems perspective. J Neurosci 22: 3312–3320.
Gawin FH (1991). Cocaine addiction: psychology and neurophysiology. Science 251: 1580–1586.
Ghasemzadeh MB, Permenter LK, Lake R, Worley PF, Kalivas PW (2003). Homer1 proteins and AMPA receptors modulate cocaine-induced behavioural plasticity. Eur J Neurosci 18: 1645–1651.
Ghitza UE, Fabbricatore AT, Prokopenko V, Pawlak AP, West MO (2003). Persistent cue-evoked activity of accumbens neurons after prolonged abstinence from self-administered cocaine. J Neurosci 23: 7239–7245.
Green JD (1958). A simple microelectrode for recording from the central nervous system. Nature 182: 962.
Grimm JW, Hope BT, Wise RA, Shaham Y (2001). Neuroadaptation. Incubation of craving after withdrawal. Nature 412: 141–142.
Ito R, Robbins TW, Everitt BJ (2004). Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci 7: 389–397.
Kalivas PW, McFarland K, Bowers S, Szumlinski K, Xi ZX, Baker D (2003). Glutamate transmission and addiction to cocaine. Ann NY Acad Sci 1003: 169–175.
Kelley AE (2004). Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev 27: 765–766.
Koob GF, Le Moal M (2001). Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharm 24: 97–129.
Koob GF, Le Moal M (1997). Drug abuse: hedonic homeostatic dysregulation. Science 278: 52–57.
Lu L, Grimm JW, Dempsey J, Shaham Y (2004). Cocaine seeking over extended withdrawal periods in rats: different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology 176: 101–108.
Lu L, Grimm JW, Shaham Y, Hope BT (2003). Molecular neuroadaptations in the accumbens and ventral tegmental area during the first 90 days of forced abstinence from cocaine self-administration in rats. J Neurochem 85: 1604–1613.
McFarland K, Kalivas PW (2001). The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 21: 8655–8663.
Nicolelis MAL (1999). Methods for Neural Ensemble Recordings. CRC Press: Boca Raton, FL. 257pp.
Nicolelis MAL, Ghazanfar AA, Faggin BM, Votaw S, Oliveira LMO (1997). Reconstructing the engram: simultaneous, multisite, may single neuron recordings. Neuron 18: 529–537.
O'Brien CP (1997). A range of research-based pharmacotherapies for addiction. Science 278: 66–70.
Paxinos G, Watson C (1998). The Rat Brain in Stereotaxic Coordinates. Academic Press: San Diego, CA. 256pp.
Peoples LL, Gee F, Bibi R, West MO (1998a). Phasic firing time locked to cocaine self-infusion and locomotion: dissociable firing patterns of single nucleus accumbens neurons in the rat. J Neursci 18: 7588–7598.
Peoples LL, Uzwiak AJ, Guyette FX, West MO (1998b). Tonic inhibition of single nucleus accumbens neurons in the rat: a predominant but not exclusive firing pattern induced by cocaine self-administration sessions. Neuroscience 86: 13–22.
Peoples LL, West MO (1996). Phasic firing of single neurons in the rat nucleus accumbens correlated with the timing of intravenous cocaine self-administration. J Neurosci 16: 3459–3473.
Robinson TE, Gorny G, Mitton E, Kolb B (2001). Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse 39: 257–266.
Shaham Y, Shalev U, Lu L, De Wit H, Stewart J (2003). The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology 168: 3–20.
Toda S, McGinty JF, Kalivas PW (2002). Repeated cocaine administration alters the expression of genes in corticolimbic circuitry after a 3-week withdrawal: a DNA macroarray study. J Neurochem 82: 1290–1299.
Tran-Nguyen LTL, Fuchs RA, Coffey GP, Baker DA, O'Dell LE, Neisewander JL (1998). Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacology 19: 48–59.
Uzwiak AJ, Guyette FX, West MO, Peoples LL (1997). Neurons in accumbens subterritories of the rat: phasic firing time-locked within seconds of intravenous cocaine infusion. Brain Res 767: 363–369.
Wise RA (1984). Neural mechanisms of the reinforcing actions of cocaine. NIDA Res Mono 50: 15–33.
Xi ZX, Ramamoorthy S, Shen H, Lake R, Samuvel DJ, Kalivas PW (2003). GABA transmission in the nucleus accumbens is altered after withdrawal from repeated cocaine. J Neurosci 23: 3498–3505.
Zahm DS (1999). Functional–anatomical implications of the nucleus accumbens core and shell subterritories. Ann NY Acad Sci 877: 113–128.
Zahm DS, Brog JS (1992). On the significance of subterritories in the ‘accumbens’ part of the rat striatum. Neurosci 50: 751–767.
Zahm DS, Heimer L (1993). Specificity in the efferent projections of the nucleus accumbens in the rat: comparison of the rostral pole projection patterns with those of the core and shell. J Comp Neurol 327: 220–232.
Acknowledgements
This research was supported by NIDA DA14339 to RMC and NIDA Training Grant DA07244 to JAH. We thank Yvette Peters and Robert Wheeler for helpful suggestions on the manuscript and Jay Elliott for help with data analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hollander, J., Carelli, R. Abstinence from Cocaine Self-Administration Heightens Neural Encoding of Goal-Directed Behaviors in the Accumbens. Neuropsychopharmacol 30, 1464–1474 (2005). https://doi.org/10.1038/sj.npp.1300748
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.npp.1300748
Keywords
This article is cited by
-
A sex-dependent role for the prelimbic cortex in impulsive action both before and following early cocaine abstinence
Neuropsychopharmacology (2021)
-
Role of nucleus accumbens core but not shell in incubation of methamphetamine craving after voluntary abstinence
Neuropsychopharmacology (2020)
-
Incubation of Accumbal Neuronal Reactivity to Cocaine Cues During Abstinence Predicts Individual Vulnerability to Relapse
Neuropsychopharmacology (2018)
-
Dynamic Alterations of Rat Nucleus Accumbens Dendritic Spines over 2 Months of Abstinence from Extended-Access Cocaine Self-Administration
Neuropsychopharmacology (2017)
-
Synaptic mechanisms underlying persistent cocaine craving
Nature Reviews Neuroscience (2016)