Abstract
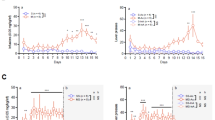
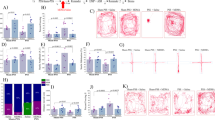
Although amphetamine-derived stimulants are widely associated with neurotoxicity, it is poorly understood whether extended exposure to such drugs produces lasting effects on neurocognitive function. This study investigates whether chronically self-administered d-amphetamine, methamphetamine (MA), or methylenedioxymethamphetamine (MDMA) leads to residual deficits in a rodent test of sustained visual attention and impulsivity. Rats were trained on a five-choice serial reaction time task and subsequently trained to self-administer d-amphetamine, MA, or MDMA (all 50 μg/infusion), intravenously, for 3 weeks. Effects on performance were evaluated 24 h after drug discontinuation and for several weeks thereafter, including various challenge sessions to increase the attentional demands of the task. The results indicate divergent patterns of self-administration among the three drugs tested with increasing rates of intake evident in rats self-administering amphetamine, but not MA, and widely fluctuating rates in the MDMA group. Withdrawal of MA resulted in severe behavioral disturbances, with significant effects on accuracy, omissions, response latency, and impulsivity that lasted up to 2 weeks in some cases. Amphetamine and MDMA withdrawal were associated with similar, but shorter-lasting effects on performance. However, when challenged with a high event rate session 6 weeks after drug discontinuation, rats previously exposed to MDMA continued to show deficits in the accuracy and speed of responding. These findings show that amphetamine-derived stimulants have both short- and long-term consequences for psychomotor functioning. The demonstration of residual deficits in rats chronically exposed to MDMA raises some concern about the potential harm caused by this drug in human ecstasy users.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ahmed SH, Koob GF (1999). Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology 146: 303–312.
Ahmed SH, Walker JR, Koob GF (2000). Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology 22: 413–421.
Beck J, Rosenbaum M (1994). Pursuit of Ecstasy: The MDMA Experience. State University of New York Press: Albany.
Bolla KI, McCann UD, Ricaurte GA (1998). Memory impairment in abstinent MDMA (‘Ecstasy’) users. Neurology 51: 1532–1537.
Caine SB, Lintz R, Koob GF (1992). Intravenous drug self-administration techniques in animals. In: Sahgal A (ed). Behavioral Neuroscience, A Practical Approach, Vol 1. Oxford University Press: Oxford. pp 117–143.
Cardinal RN, Aitken MRF (2001). Whisker, version 2.2, computer software. http://www.whisker.control.com.
Carli M, Samanin R (1992). Serotonin2 receptor agonists and serotonergic anorectic drugs affect rats' performance differently in a five-choice serial reaction time task. Psychopharmacology 106: 228–234.
Chudasama Y, Passetti F, Desai A, Rhodes S, Lopian D, Robbins TW (2003). Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res 146: 105–119.
Cornish JL, Shahnawaz Z, Thompson MR, Wong S, Morley KC, Hunt GE et al (2003). Heat increases 3,4-methylenedioxymethamphetamine self-administration and social effects in rats. Eur J Pharmacol 482: 339–341.
Dalley JW, Theobald DE, Eagle DM, Passetti F, Robbins TW (2002). Deficits in impulse control associated with tonically-elevated serotonergic function in rat prefrontal cortex. Neuropsychopharmacology 26: 716–728.
Dalley JW, Theobald DE, Berry D, Milstein J, Lääne K, Everitt BJ et al (2005a). Cognitive sequelae of intravenous amphetamine self-administration in rats: evidence for selective effects on attentional performance. Neuropsychopharmacology 30: 525–537.
Dalley JW, Lääne K, Pena Y, Theobald DE, Everitt BJ, Robbins TW (2005b). Attention and motivational deficits in rats withdrawn from intravenous self-administration of cocaine or heroin. Psychopharmacology 182: 579–587.
Ernst T, Chang L, Leonido-Yee M, Speck O (2000). Evidence for long-term neurotoxicity associated with methamphetamine abuse: a 1H MRS study. Neurology 54: 1344–1349.
Evenden JL (1999). Impulsivity: a discussion of clinical and experimental findings. J Psychopharmacol 137: 410–414.
Fantegrossi WE, Ullrich T, Rice KC, Woods JH, Winger G (2002). 3,4-Methylenedioxymethamphetamine (MDMA, ‘ecstasy’) and its stereoisomers as reinforcers in rhesus monkeys: serotonergic involvement. Psychopharmacology 161: 356–364.
Fantegrossi WE, Woolverton WL, Kilbourn M, Sherman P, Yuan J, Hatzidimitriou G et al (2004). Behavioral and neurochemical consequences of long-term intravenous self-administration of MDMA and its enantiomers by rhesus monkeys. Neuropsychopharmacology 29: 1270–1281.
Gobert A, Millan MJ (1999). Serotonin (5-HT)2A receptor activation enhances dialysate levels of dopamine and noradrenaline, but not 5-HT, in the frontal cortex of freely-moving rats. Neuropharmacology 38: 315–317.
Gouzoulis-Mayfrank E, Daumann J, Tuchtenhagen F, Pelz S, Becker S, Kunert HJ et al (2000). Impaired cognitive performance in drug free users of recreational ecstasy (MDMA). J Neurol Neurosurg Psychiatry 68: 719–725.
Gresch PJ, Smith RL, Barrett RJ, Sanders-Bush E (2005). Behavioral tolerance to lysergic acid diethylamide is associated with reduced serotonin-2A receptor signaling in rat cortex. Neuropsychopharmacology 30: 1693–1702.
Harrison AA, Everitt BJ, Robbins TW (1999). Central 5-HT depletion enhances impulsive responding without affecting the accuracy of attentional performance: interactions with dopaminergic mechanisms. Psychopharmacology 133: 329–342.
Harvey DC, Lcan G, Tanious SP, Melega WP (2000). Recovery from methamphetamine induced long-term nigrostriatal dopaminergic deficits without substantia nigra cell loss. Brain Res 871: 259–270.
Hester R, Garavan H (2004). Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci 24: 11017–11022.
Jentsch JD, Taylor JR (1999). Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology 146: 373–390.
Johanson C-E, Frey KA, Lundahl LH, Keenan P, Lockhart N, Roll J et al (2006). Cognitive function and nigrostriatal markers in abstinent methamphetamine abusers. Psychopharmacology 185: 327–338.
Kalechstein AD, Newton TF, Green M (2003). Methamphetamine dependence is associated with neurocognitive impairment in the initial phases of abstinence. J Neuropsychiatry Clin Neurosci 15: 215–220.
Keppel G (1991). Design and Analysis. Prentice-Hall: Englewood Cliffs, NJ.
Khantzian EJ (1985). The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. Am J Psychiatry 142: 1259–1264.
Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L (2006). Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology 186: 48–53.
Krystal JH, Price LH, Opsahl C, Ricaurte GA, Heninger GR (1992). Chronic 3,4-methylenedioxymethamphetamine (MDMA) use: effects on mood and neuropsychological function. Am J Drug Alcohol Abuse 18: 331–341.
Levin FR, Kleber HD (1995). Attention-deficit hyperactivity disorder and substance abuse: relationships and implications for treatment. Harv Rev Psychiatry 2: 246–258.
Lin D, Koob GF, Markou A (1999). Differential effects of withdrawal from chronic amphetamine or fluoxetine administration on brain stimulation reward in the rat—interactions between the two drugs. Psychopharmacology 145: 283–294.
London ED, Berman SM, Voytek B, Simon SL, Mandelkern MA, Monterosso J et al (2005). Cerebral metabolic dysfunction and impaired vigilance in recently abstinent methamphetamine abusers. Biol Psychiatry 58: 770–778.
Lorez H (1981). Fluorescence histochemistry indicates damage of striatal dopamine nerve terminals in rats after multiple doses of methamphetamine. Life Sci 28: 911–916.
McCann UD, Ricaurte GA (2004). Amphetamine neurotoxicity: accomplishments and remaining challenges. Neurosci Biobehav Rev 27: 821–826.
McCann UD, Szabo Z, Scheffel U, Dannals RF, Ricaurte GA (1998a). Positron emission tomographic evidence of toxic effect of MDMA (‘ecstasy’) on brain serotonin in human beings. Lancet 352: 1433–1437.
McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA (1998b). Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci 18: 8417–8422.
McKetin R, Mattick RP (1997). Attention and memory in illicit amphetamine users. Drug Alcohol Depend 48: 235–242.
Melega WP, Raleigh MJ, Stout DB, Lacan G, Huang SC, Phelps ME (1997). Recovery of striatal dopamine function after acute amphetamine and methamphetamine-induced neurotoxicity in the vervet monkey. Brain Res 766: 113–120.
Metzger RR, Hanson GR, Gibb JW, Fleckenstein AE (1998). 3,4-Methylenedioxymethamphetamine-induced acute changes in dopamine transporter function. Eur J Pharmacol 349: 205–210.
Montgomery C, Fisk JE, Newcombe R, Murphy PN (2006). The differential effects of ecstasy/polydrug use on executive components: shifting, inhibition, updating and access to semantic memory. Psychopharmacology 182: 262–276.
Nordahl TE, Salo R, Leamon M (2003). Neuropsychological effects of chronic methamphetamine use on neurotransmitters and cognition: a review. J Neuropsychiatry Clin Neurosci 15: 317–325.
Parrot AC, Lees A, Garnham NJ, Jones M, Wesnes K (1998). Cognitive performance in recreational users of MDMA or ecstasy. J Psychopharmacology 12: 79–83.
Ratzenboeck E, Saria A, Kriechbaum N, Zernig G (2001). Reinforcing effects of MDMA (‘Ecstasy’) in drug-naïve and cocaine-trained rats. Pharmacology 62: 138–144.
Ricaurte GA, Martello AL, Katz JL, Martello MB (1992). Lasting effects of (+/−)-3,4-methylenedioxymethamphetamine (MDMA) on central serotonergic neurons in nonhuman primates: neurochemicral observations. J Pharmacol Exp Ther 261: 616–622.
Richards JB, Sabol K, de Wit H (1999). Effects of methamphetamine on the adjusting amount procedure, a model of impulsive behavior in rats. Psychopharmacology 146: 432–439.
Robbins TW (2002). The 5-choice serial reaction time task: behavioral pharmacology and functional neurochemistry. Psychopharmacology 163: 363–380.
Robinson TE, Kolb B (2004). Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology 47: 33–46.
Roiser JP, Blackwell AD, Cools R, Clark L, Rubinsztein DC, Robbins TW et al (2006). Serotonin transporter polymorphism mediates vulnerability to loss of incentive motivation following acute tryptophan depletion. Neuropsychopharmacology 31: 2264–2272.
Schenk S, Gittings D, Johnstone M, Daniela E (2003). Development, maintenance and temporal pattern of self-administration maintained by ecstasy (MDMA) in rats. Psychopharmacology 169: 21–27.
Shepard JD, Chuang DT, Shaham Y, Morales M (2006). Effect of methamphetamine self-administration on tyrosine hydroxylase and dopamine transporter levels in mesolimbic and nigrostriatal dopamine pathways in the rat. Psychopharmacology 185: 505–513.
Seiden LS, Fischman MW, Schuster CR (1976). Long-term methamphetamine induced changes in brain catecholamines in tolerant rhesus monkeys. Drug Alcohol Depend 1: 215–219.
Segal DS, Kuczenski R, O’Neil ML, Melega WP, Cho AK (2005). Prolonged exposure of rats to intravenous methamphetamine: behavioral and neurochemical characterisation. Psychopharmacology 180: 501–512.
Semple DM, Ebmeier KP, Glabus MF, O’Carroll RE, Johnstone EC (1999). Reduced in vivo binding to the serotonin transporter in the cerebral cortex of MDMA (‘ecstasy’) users. Br J Psychiatry 175: 63–69.
Sim T, Simon SL, Domier CP, Richardson K, Rawson RA, Ling W (2002). Cognitive deficits among methamphetamine users with attention deficit hyperactivity disorder symptomatology. J Addict Dis 21: 75–89.
Simon SL, Domier CP, Carnell J, Brethen P, Rawson R, Ling W (2000). Cognitive impairment in individuals currently using methamphetamine. Am J Addict 9: 222–231.
Schmidt CJ, Sullivan CK, Fadayel GN (1994). Blockade of striatal 5-HT2 receptors reduced the increase in extracellular concentrations of dopamine produced by the amphetamine analogue 3,4-methylenedioxymethamphetamine. J Neurochem 62: 1382–1389.
Stefanski R, Lee S-H, Yasar S, Cadet JL, Goldberg SR (2002). Lack of persistent changes in the dopaminergic system of rats withdrawn from methamphetamine self-administration. Eur J Pharmacol 439: 59–68.
Sumnall HR, Cole JC (2005). Self-reported depressive symptomatology in community samples of polysubstance misusers who report Ecstasy use: a meta-analysis. J Psychopharmacol 19: 84–92.
Thomasius R, Petersen K, Buchert R, Andersen B, Zapletalova P, Wartberg L et al (2003). Mood, cognition and serotonin transporter availability in current and former ecstasy (MDMA) users. Psychopharmacology 167: 85–96.
Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y et al (2004). Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci 24: 6028–6036.
Trigo JM, Panayi F, Soria G, Maldonado R, Robledo P (2006). A reliable model of intravenous MDMA self-administration in naive rats. Psychopharmacology 184: 212–220.
Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M et al (2001). Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci 21: 9414–9418.
Volkow ND, Fowler JS, Wang G-J, Hitzemann R, Logan J, Schlyer DJ et al (1993). Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse 14: 169–177.
Von Sydow K, Lieb R, Pfister H, Hofler M, Wittchen HU (2002). Use, abuse and dependence of ecstasy and related drugs in adolescents and young adults—a transient phenomenon? Results from a longitudinal community study. Drug Alcohol Depend 66: 147–159.
Wang GJ, Volkow ND, Chang L, Miller E, Sedler M, Hitzemann R et al (2004). Partial recovery of brain metabolism in methamphetamine abusers after protracted abstinence. Am J Psychiatry 161: 242–248.
Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM et al (1996). Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Neurosci 2: 699–703.
Winstanley CA, Dalley JW, Theobald DE, Robbins TW (2004). Fractionating impulsivity: contrasting effects of central 5-HT depletion on different measures of impulsive behavior. Neuropsychopharmacology 29: 1331–1343.
Winter JC (1971). Tolerance to the behavioral effect of lysergic acid diethylamide and cross-tolerance to mescaline in the rat: absence of a metabolic component. J Pharmacol Exp Ther 178: 625–630.
Woolverton WL, Ricaurte GA, Forno LS, Seiden LS (1989). Long-term effects of chronic methamphetamine administration in rhesus monkeys. Brain Res 486: 73–78.
Yan Q, Reith ME, Yan S (2000). Enhanced accumbal dopamine release following 5-HT(2A) receptor stimulation in rats pretreated with intermittent cocaine. Brain Res 863: 254–258.
Acknowledgements
This research was supported by a consortium joint award from the UK Medical Research Council (MRC), and Wellcome Trust within the University of Cambridge Behavioural and Clinical Neuroscience Institute (BCNI), and by an MRC Pathfinder award to JWD, BJE and TWR (G0401068). YP was supported by a predoctoral FI scholarship from Generalitat de Catalunya.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dalley, J., Lääne, K., Theobald, D. et al. Enduring Deficits in Sustained Visual Attention during Withdrawal of Intravenous Methylenedioxymethamphetamine Self-Administration in Rats: Results from a Comparative Study with d-Amphetamine and Methamphetamine. Neuropsychopharmacol 32, 1195–1206 (2007). https://doi.org/10.1038/sj.npp.1301220
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.npp.1301220
Keywords
This article is cited by
-
Effects of Methamphetamine Exposure on Fear Learning and Memory in Adult and Adolescent Rats
Neurochemical Research (2019)
-
Comparison of the effects of abstinence on MDMA and cocaine self-administration in rats
Psychopharmacology (2018)
-
Chemogenetic Activation of Midbrain Dopamine Neurons Affects Attention, but not Impulsivity, in the Five-Choice Serial Reaction Time Task in Rats
Neuropsychopharmacology (2017)
-
Memory impairment and alterations in prefrontal cortex gamma band activity following methamphetamine sensitization
Psychopharmacology (2015)
-
In vivo potency and efficacy of the novel cathinone α-pyrrolidinopentiophenone and 3,4-methylenedioxypyrovalerone: self-administration and locomotor stimulation in male rats
Psychopharmacology (2015)