Abstract
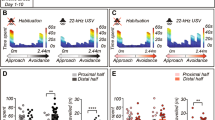
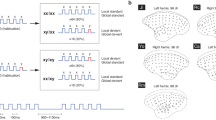
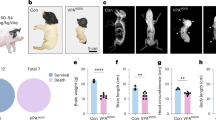
A core feature of autism spectrum disorders is the impairment in social interactions. Among other brain regions, a deficit in amygdala processing has been suggested to underlie this impairment, but whether the amygdala is processing fear abnormally in autism, is yet not clear. We used the valproic acid (VPA) rat model of autism to (a) screen for autism-like symptoms in rats, (b) test for alterations in amygdala-dependent fear processing, and (c) evaluate neuronal reactivity and synaptic plasticity in the lateral amygdala by means of in vitro single-cell electrophysiological recordings. VPA-treated animals displayed several symptoms common to autism, among them impaired social interactions and increased repetitive behaviors. Furthermore, VPA-treated rats were more anxious and exhibited abnormally high and longer lasting fear memories, which were overgeneralized and harder to extinguish. On the cellular level, the amygdala was hyperreactive to electrical stimulation and displayed boosted synaptic plasticity as well as a deficit in inhibition. We show for the first time enhanced, overgeneralized and resistant conditioned fear memories in an animal model of autism. Such hyperfear could be caused by the hyperreactivity and hyperplasticity found in the lateral amygdala, which may in turn be due to a deficit in the inhibitory system of the amygdala. We hypothesize an ‘aversive world’ syndrome that could, even if not a primary cause of the disorder itself, underlie some core symptoms in autism, such as impairments in social interactions and resistance to rehabilitation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Adolphs R, Sears L, Piven J (2001). Abnormal processing of social information from faces in autism. J Cogn Neurosci 13: 232–240.
Amaral DG, Bauman MD, Schumann CM (2003). The amygdala and autism: implications from non-human primate studies. Genes Brain Behav 2: 295–302.
Arndt TL, Stodgell CJ, Rodier PM (2005). The teratology of autism. Int J Dev Neurosci 23: 189–199.
American Psychiatric Association (1994). Diagnostic and Statistical Manual of Mental Disorders, 4th edn. Washington, DC.
Aylward EH, Minshew NJ, Goldstein G, Honeycutt NA, Augustine AM, Yates KO et al (1999). MRI volumes of amygdala and hippocampus in non-mentally retarded autistic adolescents and adults. Neurology 53: 2145–2150.
Bachevalier J (1994). Medial temporal lobe structures and autism: a review of clinical and experimental findings. Neuropsychologia 32: 627–648.
Bachevalier J, Loveland KA (2006). The orbitofrontal-amygdala circuit and self-regulation of social-emotional behavior in autism. Neurosci Biobehav Rev 30: 97–117.
Barad M (2006). Is extinction of fear erasure or inhibition? Why both, of course. Learn Mem 13: 108–109.
Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, Williams SC (2000). The amygdala theory of autism. Neurosci Biobehav Rev 24: 355–364.
Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A et al (1999). Social intelligence in the normal and autistic brain: an fMRI study. Eur J Neurosci 11: 1891–1898.
Bauer EP, Schafe GE, LeDoux JE (2002). NMDA receptors and L-type voltage-gated calcium channels contribute to long-term potentiation and different components of fear memory formation in the lateral amygdala. J Neurosci 22: 5239–5249.
Bernier R, Dawson G, Panagiotides H, Webb S (2005). Individuals with autism spectrum disorder show normal responses to a fear potential startle paradigm. J Autism Dev Disord 35: 575–583.
Bissiere S, Humeau Y, Luthi A (2003). Dopamine gates LTP induction in lateral amygdala by suppressing feedforward inhibition. Nat Neurosci 6: 587–592.
Christianson AL, Chesler N, Kromberg JG (1994). Fetal valproate syndrome: clinical and neuro-developmental features in two sibling pairs. Dev Med Child Neurol 36: 361–369.
Collins DR, Pare D (2000). Differential fear conditioning induces reciprocal changes in the sensory responses of lateral amygdala neurons to the CS(+) and CS(−). Learn Mem 7: 97–103.
Corbett BA, Mendoza S, Abdullah M, Wegelin JA, Levine S (2006). Cortisol circadian rhythms and response to stress in children with autism. Psychoneuroendocrinology 31: 59–68.
Critchley H, Daly E, Phillips M, Brammer M, Bullmore E, Williams S et al (2000). Explicit and implicit neural mechanisms for processing of social information from facial expressions: a functional magnetic resonance imaging study. Hum Brain Mapp 9: 93–105.
Davis M, Whalen PJ (2001). The amygdala: vigilance and emotion. Mol Psychiatry 6: 13–34.
Evans DW, Canavera K, Kleinpeter FL, Maccubbin E, Taga K (2005). The fears, phobias and anxieties of children with autism spectrum disorders and down syndrome: comparisons with developmentally and chronologically age matched children. Child Psychiatry Hum Dev 36: 3–26.
Falls WA, Miserendino MJ, Davis M (1992). Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci 12: 854–863.
Gillott A, Furniss F, Walter A (2001). Anxiety in high-functioning children with autism. Autism 5: 277–286.
Hirstein W, Iversen P, Ramachandran VS (2001). Autonomic responses of autistic children to people and objects. Proc Biol Sci 268: 1883–1888.
Ingram JL, Peckham SM, Tisdale B, Rodier PM (2000). Prenatal exposure of rats to valproic acid reproduces the cerebellar anomalies associated with autism. Neurotoxicol Teratol 22: 319–324.
Isoardi NA, Martijena ID, Carrer HF, Molina VA (2004). Increased fear learning coincides with neuronal dysinhibition and facilitated LTP in the basolateral amygdala following benzodiazepine withdrawal in rats. Neuropsychopharmacology 29: 1852–1864.
Kahne D, Tudorica A, Borella A, Shapiro L, Johnstone F, Huang W et al (2002). Behavioral and magnetic resonance spectroscopic studies in the rat hyperserotonemic model of autism. Physiol Behav 75: 403–410.
Kanner L (1943). Autistic disturbances of affective contact. Nerv Child 2: 217–250.
Kemper TL, Bauman M (1998). Neuropathology of infantile autism. J Neuropathol Exp Neurol 57: 645–652.
Kling A, Brothers L (1992). The Amygdala and Social Behavior. Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. J. Aggleton, Wiley: New York.
La Mendola D, Markram K, Rinaldi T, Sandi C, Markram H (2006). Hyper-fear, reduced fear extinction and weakened inhibition in the amygdala in an animal model of autism. FENS (Abstract, 3, A026.12).
LeDoux J (2003). The emotional brain, fear, and the amygdala. Cell Mol Neurobiol 23: 727–738.
LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM (1990). The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci 10: 1062–1069.
Li XF, Armony JL, LeDoux JE (1996). GABAA and GABAB receptors differentially regulate synaptic transmission in the auditory thalamo-amygdala pathway: an in vivo microiontophoretic study and a model. Synapse 24: 115–124.
Lu KT, Walker DL, Davis M (2001). Mitogen-activated protein kinase cascade in the basolateral nucleus of amygdala is involved in extinction of fear-potentiated startle. J Neurosci 21: RC162.
Markram K, La Mendola D, Rinaldi T, Sandi C, Markram H (2005). Enhanced fear and reduced fear extinction in an animal model of autism. Soc Neurosci (Abstract, 448.3).
McAlonan GM, Daly E, Kumari V, Critchley HD, van Amelsvoort T, Suckling J et al (2002). Brain anatomy and sensorimotor gating in Asperger's syndrome. Brain 125: 1594–1606.
Meyer-Lindenberg A, Hariri AR, Munoz KE, Mervis CB, Mattay VS, Morris CA et al (2005). Neural correlates of genetically abnormal social cognition in Williams syndrome. Nat Neurosci 8: 991–993.
Moore SJ, Turnpenny P, Quinn A, Glover S, Lloyd DJ, Montgomery T et al (2000). A clinical study of 57 children with fetal anticonvulsant syndromes. J Med Genet 37: 489–497.
Morris RG, Garrud P, Rawlins JN, O'Keefe J (1982). Place navigation impaired in rats with hippocampal lesions. Nature 297: 681–683.
Muris P, Steerneman P, Merckelbach H, Holdrinet I, Meesters C (1998). Comorbid anxiety symptoms in children with pervasive developmental disorders. J Anxiety Disord 12: 387–393.
Narita N, Kato M, Tazoe M, Miyazaki K, Narita M, Okado N (2002). Increased monoamine concentration in the brain and blood of fetal thalidomide- and valproic acid-exposed rat: putative animal models for autism. Pediatr Res 52: 576–579.
Newman JD, Bachevalier J (1997). Neonatal ablations of the amygdala and inferior temporal cortex alter the vocal response to social separation in rhesus macaques. Brain Res 758: 180–186.
Pelphrey K, Adolphs R, Morris JP (2004). Neuroanatomical substrates of social cognition dysfunction in autism. Ment Retard Dev Disabil Res Rev 10: 259–271.
Perry W, Minassian A, Lopez B, Maron L, Lincoln A (2007). Sensorimotor gating deficits in adults with autism. Biol Psychiatry 61: 482–486.
Phillips RG, LeDoux JE (1992). Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci 106: 274–285.
Pierce K, Muller RA, Ambrose J, Allen G, Courchesne E (2001). Face processing occurs outside the fusiform ‘face area’ in autism: evidence from functional MRI. Brain 124: 2059–2073.
Quirk GJ, Likhtik E, Pelletier JG, Pare D (2003). Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci 23: 8800–8807.
Quirk GJ, Repa C, LeDoux JE (1995). Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron 15: 1029–1039.
Rasalam AD, Hailey H, Williams JH, Moore SJ, Turnpenny PD, Lloyd DJ et al (2005). Characteristics of fetal anticonvulsant syndrome associated autistic disorder. Dev Med Child Neurol 47: 551–555.
Repa JC, Muller J, Apergis J, Desrochers TM, Zhou Y, LeDoux JE (2001). Two different lateral amygdala cell populations contribute to the initiation and storage of memory. Nat Neurosci 4: 724–731.
Rinaldi T, Kulangara K, Antoniello K, Markram H (submitted). Elevated NMDA receptor levels and enhanced postsynaptic long term potentiation induced by prental exposure to valproic acid. Proc Natl Acad Sci USA (in press).
Ritvo ER, Freeman BJ, Scheibel AB, Duong T, Robinson H, Guthrie D et al (1986). Lower Purkinje cell counts in the cerebella of four autistic subjects: initial findings of the UCLA-NSAC Autopsy Research Report. Am J Psychiatry 143: 862–866.
Rodier PM, Ingram JL, Tisdale B, Croog VJ (1997). Linking etiologies in humans and animal models: studies of autism. Reprod Toxicol 11: 417–422.
Rodier PM, Ingram JL, Tisdale B, Nelson S, Romano J (1996). Embryological origin for autism: developmental anomalies of the cranial nerve motor nuclei. J Comp Neurol 370: 247–261.
Rodriguez Manzanares PA, Isoardi NA, Carrer HF, Molina VA (2005). Previous stress facilitates fear memory, attenuates GABAergic inhibition, and increases synaptic plasticity in the rat basolateral amygdala. J Neurosci 25: 8725–8734.
Rogan MT, Staubli UV, LeDoux JE (1997). Fear conditioning induces associative long-term potentiation in the amygdala. Nature 390: 604–607.
Roozendaal B, Sapolsky RM, McGaugh JL (1998). Basolateral amygdala lesions block the disruptive effects of long-term adrenalectomy on spatial memory. Neuroscience 84: 453–465.
Rosenkranz JA, Moore H, Grace AA (2003). The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci 23: 11054–11064.
Schneider T, Przewlocki R (2005). Behavioral alterations in rats prenatally exposed to valproic acid: animal model of autism. Neuropsychopharmacology 30: 80–89.
Schneider T, Turczak J, Przewlocki R (2006). Environmental enrichment reverses behavioral alterations in rats prenatally exposed to valproic Acid: issues for a therapeutic approach in autism. Neuropsychopharmacology 31: 36–46.
Schultz RT (2005). Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int J Dev Neurosci 23: 125–141.
Schumann CM, Amaral DG (2006). Stereological analysis of amygdala neuron number in autism. J Neurosci 26: 7674–7679.
Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH et al (2004). The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J Neurosci 24: 6392–6401.
Shaban H, Humeau Y, Herry C, Cassasus G, Shigemoto R, Ciocchi S et al (2006). Generalization of amygdala LTP and conditioned fear in the absence of presynaptic inhibition. Nat Neurosci 9: 1028–1035.
Shekhar A, Truitt W, Rainnie D, Sajdyk T (2005). Role of stress, corticotrophin releasing factor (CRF) and amygdala plasticity in chronic anxiety. Stress 8: 209–219.
Sotres-Bayon F, Bush DE, LeDoux JE (2004). Emotional perseveration: an update on prefrontal-amygdala interactions in fear extinction. Learn Mem 11: 525–535.
Sotres-Bayon F, Cain CK, Ledoux JE (2006). Brain mechanisms of fear extinction: historical perspectives on the contribution of prefrontal cortex. Biol Psychiatry 60: 329–336.
Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA et al (2002). Brain structural abnormalities in young children with autism spectrum disorder. Neurology 59: 184–192.
Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG (2002). Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry 59: 1027–1034.
Sweeten TL, Posey DJ, Shekhar A, McDougle CJ (2002). The amygdala and related structures in the pathophysiology of autism. Pharmacol Biochem Behav 71: 449–455.
Szinyei C, Heinbockel T, Montagne J, Pape HC (2000). Putative cortical and thalamic inputs elicit convergent excitation in a population of GABAergic interneurons of the lateral amygdala. J Neurosci 20: 8909–8915.
Takagi M, Yamamoto C (1981). The long-lasting inhibition recorded in vitro from the lateral nucleus of the amygdala. Brain Res 206: 474–478.
Tillfors M (2004). Why do some individuals develop social phobia? A review with emphasis on the neurobiological influences. Nord J Psychiatry 58: 267–276.
Towbin KE, Pradella A, Gorrindo T, Pine DS, Leibenluft E (2005). Autism spectrum traits in children with mood and anxiety disorders. J Child Adolesc Psychopharmacol 15: 452–464.
Vorhees CV (1987a). Behavioral teratogenicity of valproic acid: selective effects on behavior after prenatal exposure to rats. Psychopharmacology (Berl) 92: 173–179.
Vorhees CV (1987b). Teratogenicity and developmental toxicity of valproic acid in rats. Teratology 35: 195–202.
Wagner GC, Reuhl KR, Cheh M, McRae P, Halladay AK (2006). A new neurobehavioral model of autism in mice: pre- and postnatal exposure to sodium valproate. J Autism Dev Disord 36: 779–793.
Washburn MS, Moises HC (1992a). Electrophysiological and morphological properties of rat basolateral amygdaloid neurons in vitro. J Neurosci 12: 4066–4079.
Washburn MS, Moises HC (1992b). Inhibitory responses of rat basolateral amygdaloid neurons recorded in vitro. Neuroscience 50: 811–830.
Williams G, King J, Cunningham M, Stephan M, Kerr B, Hersh JH (2001). Fetal valproate syndrome and autism: additional evidence of an association. Dev Med Child Neurol 43: 202–206.
Williams PG, Hersh JH (1997). A male with fetal valproate syndrome and autism. Dev Med Child Neurol 39: 632–634.
Woolley ML, Haman M, Higgins GA, Ballard TM (2006). Investigating the effect of bilateral amygdala lesions on fear conditioning and social interaction in the male Mongolian gerbil. Brain Res 1078: 151–158.
Yadin E, Friedman E, Bridger WH (1991). Spontaneous alternation behavior: an animal model for obsessive-compulsive disorder? Pharmacol Biochem Behav 40: 311–315.
Author information
Authors and Affiliations
Corresponding author
Additional information
DISCLOSURE/CONFLICT OF INTEREST
The authors declare that, except for income received from the primary employer, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
Rights and permissions
About this article
Cite this article
Markram, K., Rinaldi, T., Mendola, D. et al. Abnormal Fear Conditioning and Amygdala Processing in an Animal Model of Autism. Neuropsychopharmacol 33, 901–912 (2008). https://doi.org/10.1038/sj.npp.1301453
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.npp.1301453
Keywords
This article is cited by
-
Dysregulation of the Wnt/β-catenin signaling pathway via Rnf146 upregulation in a VPA-induced mouse model of autism spectrum disorder
Experimental & Molecular Medicine (2023)
-
Saffron and crocin ameliorate prenatal valproic acid-induced autistic-like behaviors and brain oxidative stress in the male offspring rats
Metabolic Brain Disease (2023)
-
Altered Developmental Trajectory in Male and Female Rats in a Prenatal Valproic Acid Exposure Model of Autism Spectrum Disorder
Journal of Autism and Developmental Disorders (2023)
-
Generational synaptic functions of GABAA receptor β3 subunit deteriorations in an animal model of social deficit
Journal of Biomedical Science (2022)
-
Novel probiotic treatment of autism spectrum disorder associated social behavioral symptoms in two rodent models
Scientific Reports (2022)