Abstract
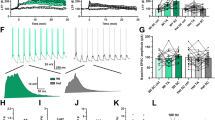
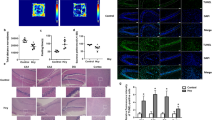
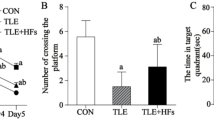
Dysfunction of hippocampal plasticity has been proposed to play a critical role in the pathophysiology of depression. However, antidepressant drug effects on synaptic plasticity and cytoskeletal remodeling remain controversial. The aim of the present study was to evaluate in animals exposed to the learned helplessness (LH) paradigm, an accepted experimental model of depression, the effect of chronic treatment with fluoxetine (FLX) on synaptic and cytoskeletal proteins known to undergo plastic changes. Synaptophysin (SYN), postsynaptic density 95 (PSD-95), axon growth-associated protein 43 (GAP-43), and cytoskeletal proteins (intermediate neurofilaments and MAP-2) were studied in the hippocampus by immunohistochemistry. Whereas LH animals treated 21 days with saline (LH-S group) displayed diminished SYN and PSD-95 immunostainings in the CA3 but not in the DG, chronic treatment with FLX not only reversed the despaired behavior induced by exposure to LH paradigm, but also fully recovered SYN and PSD-95 labeling to control values. Similar results were obtained for the axonal remodeling marker GAP-43. FLX treatment did not modify either the decreased light neurofilament subunit (NFL) observed in the hippocampus of LH animals or any other cytoskeletal protein studied. When FLX treatment was withdrawn for 90 days in those LH-FLX animals in which reversion of despair had been observed at day 25, recurrence of despaired behavior was found accompanied by decreased SYN, PSD-95, and NFL labelings. Results indicate that the synapse remodeling induced by FLX in the CA3 region could underlie its behavioral efficacy despite the absence of cytoskeletal remodeling and that the stability of synaptic changes would depend on the continuous administration of the drug.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- BDNF:
-
brain-derived neurotrophic factor
- CA3:
-
CA3 region of Ammon's horn
- DG:
-
dentate gyrus
- FLX:
-
fluoxetine
- GAP-43:
-
growth-associated protein 43
- LH:
-
learned helplessness
- MAP-2:
-
microtubule-associated protein 2
- mf:
-
mossy fibers
- MRI:
-
magnetic resonance imaging
- NF:
-
neurofilament
- NFH:
-
heavy neurofilament subunit
- NFL:
-
light neurofilament subunit
- NFM:
-
medium neurofilament subunit
- PBS:
-
phosphate-buffered saline
- PSD-95:
-
postsynaptic density 95
- pyr:
-
pyramidal cell layer
- SL:
-
stratum lucidum
- SO:
-
stratum oriens
- SR:
-
stratum radiatum
- SYN:
-
synaptophysin
References
Akhondzadeh S (1999). Hippocampal synaptic plasticity and cognition. J Clin Pharm Ther 24: 241–248.
Anisman H, Merali Z (2001). Rodent models of depression: learned helplessness induced in mice. Curr Protoc Neurosci, Unit 8.10C.
Benowitz LI, Routtenberg A (1997). GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci 20: 84–91.
Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS (2000). Hippocampal volume reduction in major depression. Am J Psychiatry 157: 115–118.
Carter AR, Chen C, Schwartz PM, Segal RA (2002). Brain-derived neurotrophic factor modulates cerebellar plasticity and synaptic ultrastructure. J Neurosci 22: 1316–1327.
Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT (2001). Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry 15: 260–265.
Cho OK, Hunt CA, Kennedy MB (1992). The rat brain postsynaptic density fraction contains a homolog of the drosophila dics-large tumor suppressor protein. Neuron 9: 929–942.
Collin C, Vicario-Abejon C, Rubio ME, Wenthold RJ, McKay RD, Segal M (2001). Neurotrophins act at presynaptic terminals to activate synapses among cultured hippocampal neurons. Eur J Neurosci 13: 1273–1282.
Czéh B, Lucassen PJ (2007). What causes the hippocampal volume decrease in depression? Are neurogenesis, glial changes and apoptosis implicated? Eur Arch Psychiatry Clin Neurosci 257: 250–260.
Czéh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kamper M et al (2001). Stress-induced changes in cerebral metabolites, hippocampal volume and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natt Acad Sci USA 98: 12796–12801.
De Foubert G, Carney SL, Robinson CS, Destexhe EJ, Tomlinson R, Hicks CA et al (2004). Fluoxetine-induced change in rat brain expression of brain-derived neurotrophic factor varies depending on length of treatment. Neuroscience 128: 597–604.
Donohue HS, Gabbott LA, Davies HS, Rodriguez JJ, Cordero MI, Sandi C et al (2006). Chronic restraint stress induces changes in synapse morphology in stratum lacunosum-moleculare CA1 rat hippocampus: a stereological and three-dimensional ultrastructural study. Neuroscience 140: 597–606.
Dulawa SC, Holick KA, Gundersen B, Hen R (2004). Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology 29: 1321–1330.
Duman RS (2002). Pathophysiology of depression: the concept of synaptic plasticity. Eur Psychiatry 17: 306–310.
Eastwood SL, Harrison PJ (2001). Synaptic pathology in the anterior cingulated cortex in schizophrenia and mood disorders. A review and western blot study of synaptophysin, GAP-43 and the complexins. Brain Res Bull 55: 569–578.
Eichenbaum H, Otto T (1992). The hippocampus—what does it do? Behav Neural Biol 57: 2–36.
Engert F, Bonhoeffer T (1999). Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature 399: 66–70.
Ferrero AJ, Cereseto M, Sifonios LL, Reines A, Peixoto E, Rubio MC et al (2007). Cytoskeleton of hippocampal neurons as a target for valproic acid in an experimental model of depression. Prog Neuropsychopharmacol Biol Psychiatry 31: 1419–1428.
Grillo CA, Piroli GG, Wood GE, Reznikov LR, McEwen BS, Reagan LP (2005). Immunocytochemical analysis of synaptic proteins provides new insights into diabetes-mediated plasticity in the rat hippocampus. Neuroscience 136: 477–486.
Hardman JG, Limbird LE (2001). Goodman and Gilman, The Pharmacological Basis of Therapeutics, 10th edn. McGraw-Hill Co: Atlampa, Mexico.
Hirao K, Hata Y, Deguchi M, Yao I, Ogura M, Rokukawa C et al (2000). Association of synapse-associated protein 90/postsynaptic density-95-associated protein (SAPAP) with neurofilaments. Genes Cells 5: 203–210.
Holderbach R, Clark K, Moreau J-L, Bischofberger J, Normann C (2006). Enhanced long-term synaptic depression in an animal model of depression. Biol Psychiat 62: 92–100.
Hu B, Nikolakopoulou AM, Cohen-Cory S (2005). BDNF stabilizes synapses and maintains the structural complexity of optic axons in vivo. Development 132: 4285–4298.
Hunt CA, Schenker LJ, Kennedy MB (1996). PSD-95 is associated with the postsynaptic density and not with the presynaptic membrane at forebrain synapses. J Neurosci 16: 1380–1388.
Iwata M, Shirayama Y, Ishida H, Kawahara R (2006). Hippocampal synapsin I, growth-associated protein-43, and microtubule-associated protein-2 immunoreactivity in learned helplessness rats and antidepressant-treated rats. Neuroscience 141: 1301–1313.
Keller MB (2001). Long-term treatment of recurrent and chronic depression. J Clin Psychiatry 62: 3–5.
Kuroda Y, McEwen BS (1998). Effect of chronic restraint stress and tianeptine on growth factors, growth-associated protein-43 and microtubule-associated protein 2 mRNA expression in the rat hippocampus. Brain Res Mol Brain Res 59: 35–39.
Li S, Reinprecht I, Fahnestock M, Racine RJ (2002). Activity-dependent changes in synaptophysin immunoreactivity in hippocampus, piriform cortex, and entorhinal cortex of the rat. Neuroscience 115: 1221–1229.
Lynch MA, Voss KL, Rodriguez J, Bliss TV (1994). Increase in synaptic vesicle proteins accompanies long-term potentiation in the dentate gyrus. Neuroscience 60: 1–5.
Magariños AM, Deslandes A, McEwen BS (1999). Effects of antidepressant and benzodiazepine treatments on the dendritic structure of CA3 pyramidal neurons after chronic stress. Eur J Pharmacol 371: 113–122.
Magariños AM, McEwen BS, Flugge G, Fuchs E (1996). Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci 16: 3534–3540.
Malberg JE, Eisch AJ, Nestler EJ, Duman RS (2000). Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 20: 9104–9110.
Markowitz JC, Milrod B (2005). Mood disorders: intrapsychic and interpersonal aspects. In: Sadock BJ, Sadock VA (eds). Comprehensive Textbook of Psychiatry. Lippincott Williams & Wilkins: Philadelphia, pp 1603–1611.
Mattson MP, Maudsley S, Martin B (2004). BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci 27: 589–594.
Mervaala E, Fohr J, Kononen M, Valkonen-Korhonen M, Vainio P, Partanen K et al (2002). Quantitative MRI of the hippocampus and amygdala in severe depression. Psychopharmacol Med 30: 117–125.
Mullany P, Lynch MA (1997). Changes in protein synthesis and synthesis of the synaptic vesicle protein, synaptophysin, in enthorinal cortex following induction of long-term potentiation in dentate gyrus: an age-related study in the rat. Neuropharmacology 36: 973–980.
Nakagawa Y, Sasaki A, Takashima T (1999). The GabaB receptor antagonist CGP36742 improves learned helplessness in rats. Eur J Pharmacol 381: 1–7.
Nemeroff CB (2007). Prevalence and management of treatment-resistant depression. J Clin Psychiatry 68: 17–25.
Neumeister A, Wood S, Bonne O, Nugent AC, Luckenbaugh DA, Young T et al (2005). Reduced hippocampal volume in unmedicated, remitted patients with major depression versus control subjects. Biol Psychiatry 57: 935–937.
Nibuya M, Morinobu S, Duman RS (1995). Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci 15: 7539–7547.
Nibuya M, Nestler EJ, Duman RS (1996). Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci 16: 2365–2372.
Paxinos G, Watson C (1986). The Rat Brain in Stereotaxis Coordinates, 4th edn. Academic Press: San Diego, CA.
Posternak MA, Zimmerman M (2007). Therapeutic effect of follow-up assessments on antidepressant and placebo response rates in antidepressant efficacy trials: meta-analysis. Br J Psychiatry 190: 287–292.
Pozzo-Miller LD, Gottschalk W, Zhang L, McDermott K, Du J, Gopalakrishnan R et al (1999). Impairments in high-frequency transmission, synaptic vesicle docking, and synaptic protein distribution in the hippocampus of BDNF knockout mice. J Neurosci 19: 4972–4983.
Ramos AJ, Tagliaferro P, Lopez EM, Pecci Saavedra JP (2000). Neuroglial interactions in a model of para-clorphenylalanine-induced serotonin depletion. Brain Res 883: 1–14.
Reinés A, Cereseto M, Ferrero A, Bonavita C, Wikinski S (2004). Neuronal cytoskeletal alterations in an experimental model of depression. Neuroscience 129: 529–538.
Rosenbrock H, Koros E, Bloching A, Podhorna J, Borsini F (2005). Effect of chronic intermittent restraint stress on hippocampal expression of markers for synaptic plasticity and progenitor cell proliferation in rats. Brain Res 1040: 55–63.
Scaccianoce S, Del Bianco P, Caricasole A, Nicoletti F, Catalani A (2003). Relationship between learning, stress, and hippocampal brain-derived neurotrophic factor. Neuroscience 121: 825–828.
Schulz DJ (2006). Plasticity and stability in neuronal output via changes in intrinsic excitability: it's what's inside that counts. J Exp Biol 209: 4821–4827.
Sheline Y, Sanghavi M, Mintun MA, Gado MH (2000). MRI studies of neuroanatomic changes in unipolar major depression: the role of stress and medical comorbidity. Biol Psychiatry 48: 791–800.
Shelton CI (2004). Long-term management of major depressive disorder: are differences among antidepressant treatments meaningful? J Clin Psychiatry 65: 29–33.
Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS (2002). Brain-derived neurotrophic factor produces antidepressant effects in behavioural models of depression. J Neurosci 22: 3251–3261.
Smith MA, Makino S, Kvetnansky R, Post RM (1995). Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci 15: 1768–1777.
Tagliaferro P, Vega MD, Evrard SG, Ramos AJ, Brusco A (2002). Alcohol exposure during adulthood induces neuronal and astroglial alterations in the hippocampal CA-1 area. Ann NY Acad Sci 965: 334–363.
Tartaglia N, Du J, Tyler WJ, Neale E, Pozzo-Miller L, Lu B (2001). Protein synthesis-dependent and -independent regulation of hippocampal synapses by brain-derived neurotrophic factor. J Biol Chem 276: 37585–37593.
Terry-Lorenzo RT, Inoue M, Connor JH, Haystead TAJ, Armbruster BN, Gupta RP et al (2000). Neurofilament-L is a protein phosphatase-1-binding protein associated with neuronal plasma membrane and post-synaptic density. J Biol Chem 275: 2439–2446.
Thome J, Pesold B, Baader M, Hu M, Gewirtz JC, Duman RS et al (2001). Stress differentially regulates synaptophysin and synaptotagmin expression in hippocampus. Biol Psychiatry 50: 809–812.
Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D (1999). LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature 402: 421–425.
Tyler WJ, Pozzo-Miller LD (2001). BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J Neurosci 21: 4249–4258.
Videbech P, Ravnkilde B (2004). Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry 161: 1957–1966.
Vollmayr B, Henn FA (2001). Learned helplessness in the rat: improvements in validity and reliability. Brain Res Protoc 8: 1–7.
Watanabe Y, Gould E, McEwen BS (1992). Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res 588: 341–345.
World Health Organization (2001). The World Health Report 2001. http://www.who.int/mental_health/management/depression/definition/en/print.html.
Xu H, He J, Richardson JS, Li XM (2004). The response of synaptophysin and microtubule-associated protein 1 to restraint stress in rat hippocampus and its modulation by venlafaxine. J Neurochem 91: 1380–1388.
Zazpe A, Artaiz I, Labeaga L, Lucero ML, Orjales A (2007). Reversal of learned helplessness by selective serotonin reuptake inhibitors in rats is not dependent on 5-HT availability. Neuropharmacology 52: 975–984.
Acknowledgements
SW and AR are Investigators from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). This work was supported by grants from Universidad de Buenos Aires (UBACYT M-013), Consejo Nacional de Investigaciones Científicas y Técnicas (PIP5870), and Agencia Nacional de Promoción Científica y Técnica (PICT 05-11102, PICT 31953 and PICT 34397), Argentina.
Author information
Authors and Affiliations
Corresponding author
Additional information
DISCLOSURE/CONFLICT OF INTEREST
The author(s) declare that, except for income received from my primary employer, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest. No author has conflict of interests related to the present research.
Rights and permissions
About this article
Cite this article
Reinés, A., Cereseto, M., Ferrero, A. et al. Maintenance Treatment with Fluoxetine is Necessary to Sustain Normal Levels of Synaptic Markers in an Experimental Model of Depression: Correlation with Behavioral Response. Neuropsychopharmacol 33, 1896–1908 (2008). https://doi.org/10.1038/sj.npp.1301596
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.npp.1301596
Keywords
This article is cited by
-
Antidepressants enter cells, organelles, and membranes
Neuropsychopharmacology (2024)
-
Structural connectivity and subcellular changes after antidepressant doses of ketamine and Ro 25-6981 in the rat: an MRI and immuno-labeling study
Brain Structure and Function (2021)
-
Buyang Huanwu decoction facilitates neurorehabilitation through an improvement of synaptic plasticity in cerebral ischemic rats
BMC Complementary and Alternative Medicine (2017)
-
Enzymatic Depletion of the Polysialic Acid Moiety Associated with the Neural Cell Adhesion Molecule Inhibits Antidepressant Efficacy
Neuropsychopharmacology (2016)
-
Fluoxetine regulates mTOR signalling in a region-dependent manner in depression-like mice
Scientific Reports (2015)