Abstract
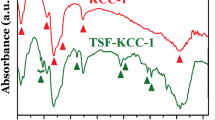
As part of a systematic study1 of Prof. Szent-Györgyi's ‘hexuronic acid’ we have investigated quantitatively the absorption spectra of hexuronic acid, glycuronic acid, galacturonic acid, tetramethyl γ-fructose, and other carbohydrate derivatives. In view of the fact that hexuronic acid has been identified with vitamin C,2 we have paid special attention to the possibility of contamination by small traces of impurity. We find that the single broad band at about 263 mμ reported qualitatively by F. P. Bowden and C. P. Snow3 is found in equal intensity with the sample of hexuronic acid supplied by Prof. Szent-Györgyi and with rigorously purified material. It appears, therefore, that this band is definitely associated with hexuronic acid. The nature of the band in methyl alcohol (c. 0.002 per cent) is indicated by the accompanying table:
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
E. L. Hirst and R. J. W. Reynolds, NATURE, April 16, 1932, p. 576.
J. L. Svirbely and A. Szent-Györgyi, ibid., p. 576.
NATURE, May 14, p. 720.
L. Kwiecinski and L. Marchlewski, Bull. Acad. Polonaise, 1927, 379.
NATURE, April 16, p. 576.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
HERBERT, R., HIRST, E. The Absorption Spectrum of Hexuronic Acid. Nature 130, 205 (1932). https://doi.org/10.1038/130205a0
Issue date:
DOI: https://doi.org/10.1038/130205a0
This article is cited by
-
Absorptionsspektren im Dienste der Vitaminforschung
Die Naturwissenschaften (1936)
-
Refractive Indices of l-Ascorbic Acid
Nature (1934)
-
Constitution of Vitamin C
Nature (1933)
-
Das vitamin C
Ergebnisse der Physiologie und Experimentellen Pharmakologie (1933)