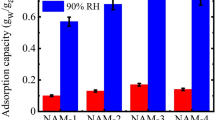
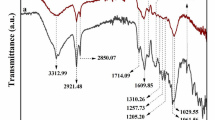
Abstract
IF a solution of a substance has a smaller surface tension than the pure solvent, the solute is adsorbed or concentrated at the surface, in accordance with Gibbs's theorem. It is therefore to be expected that the vapour pressure of the solvent of such a solution will be higher, when the concentration of the solute is made the same in the surface as in the interior, by continually renewing the surface, than when it is not.
Similar content being viewed by others
Article PDF
References
R. Pohl and P. Pringsheim, Verh. Deutsch. Phys. Ges., 15, 431 1913.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
HALBAN, H. Vapour Pressure of Potassium Amalgams. Nature 133, 463 (1934). https://doi.org/10.1038/133463c0
Issue date:
DOI: https://doi.org/10.1038/133463c0