Abstract
IT is known that, in the presence of a platinum catalyst, there is an interchange of hydrogen atoms between gaseous hydrogen and water1. Recent observations have proved that nickel can also act as a catalyst of this reaction. On the other hand, A. Farkas, L. Farkas and E. K. Rideal2 have shown that the hydrogenation of ethylene on a nickel catalyst is preceded by an interchange between the hydrogen atoms of the ethylene and the hydrogen used for hydrogenation. Afterwards it was found by the authors in collaboration with G. Ogden3 that a metallic catalyst causes a rapid exchange of hydrogen atoms between benzene and gaseous hydrogen at ordinary temperatures, while at the same time hydrogenation is very slow.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
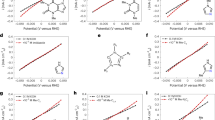
Similar content being viewed by others
References
J. Horiuti and M. Polanyi, NATURE, 132, 819, 931; 1933. Manch. Lit. Phil. Soc., 78, 47; 1934.
Chem. and Ind., 53, 489; 1934. E. K. Rideal, Chem. and Ind., 53, 610; 1934.
Trans. Far. Soc., 30, 663; 1934.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
HORIUTI, J., POLANYI, M. Catalytic Interchange of Hydrogen between Water and Ethylene and between Water and Benzene. Nature 134, 377–378 (1934). https://doi.org/10.1038/134377b0
Issue date:
DOI: https://doi.org/10.1038/134377b0
This article is cited by
-
Kinetic Study of Propylene Hydrogenation over Pt/Al2O3 by Parahydrogen-Induced Polarization
Applied Magnetic Resonance (2013)
-
Über den gegenwärtigen Stand unserer Kenntnisse vom schweren Wasserstoff und dem schweren Wasser
Protoplasma (1935)