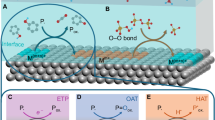
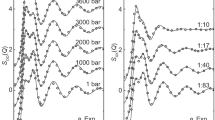
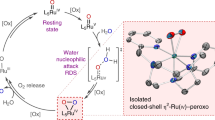
Abstract
THE account in NATURE of November 17, p. 778, by Dr. Newton Friend and Mr. S. Marks of an explosion which occurred during the preparation of oxygen from sodium peroxide and water interested us particularly, since in 1924 we had a similar experience. The oxygen was being prepared by dropping water on to solid sodium peroxide in a flask, without heating, and was being led through drying tubes to an ozoniser. The water contained a little cobalt chloride to catalyse the decomposition of the peroxide.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
CHEESMAN, G., DUNCAN, D. Oxygen Preparation from Sodium Peroxide. Nature 134, 971 (1934). https://doi.org/10.1038/134971b0
Issue date:
DOI: https://doi.org/10.1038/134971b0