Abstract
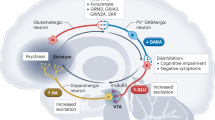
The use of animal models for the study of psychosis and new treatment development is inadequate in assessing target psychotic symptoms because animals lack an ability to use language. Despite this deficiency, new antidopaminergic antipsychotic drugs have still become available. However, even these new antipsychotics, although substantially better than the conventional compounds, do not “cure” psychosis or normalize schizophrenic symptoms. The need for new treatment strategies is apparent. The value of a human model, where language is available to describe target symptoms, is clear. Currently, there is an opportunity to use the mild psychotomimetic symptoms induced by a minimal dose of ketamine in normal humans as a model of psychosis. The mental symptoms in this model resemble some of the symptoms of schizophrenia, suggesting the additional possibility that parallel mechanisms of psychosis may occur in schizophrenia and in a ketamine state, creating a potentially viable psychosis model for pathophysiology. This paper includes arguments in support of this human model's application. Several potential outcome measures that can be used to evaluate potentially novel antipsychotics are described. This model has the potential for identifying novel therapeutics because it does not primarily utilize the dopaminergic system. Further delineation of ketamine pharmacology in humans is pivotal to the eventual application of this ketamine model in drug development.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Anand A, Berman R, Oren D, Chamey DS, Krystal JH . (1999): Attenuation of NMDA antagonist effects in healthy humans by larnotrigine, a drug that reduces glutamate release. Schizophr Res 36: 305
Andreasen NC, Rezai K, Alliger RJ, Swayze VW, Flaum M, Kirchner P, Cohen G, O'Leary DS . (1992): Hypofrontality in neuroleptic-naive patients and in patients with chronic schizophrenia. Arch Gen Psychiatry 49: 943–958
Angrist BM, Gershon S . (1970): The phenomenology of experimentally induced amphetamine psychosis: Preliminary observations. Biol Psychiatry 2: 95–107
Anis NA, Berry SC, Burton NR, Lodge D . (1983): The dissociative anesthetics ketamine and phencyclidine selectively reduce excitation of central mammalian neurons by N-methyl-D-aspartate. Br J Pharmacol 79: 5654–5675
Arnt J . (1995): Differential effects of classical and newer antipsychotics on the hypermotility induced by two dose levels of d-amphetamine. Eur J Pharmacol 283: 55–62
Beasley CM Jr, Tollefson G, Tran P, Satterlee W, Sanger G, Hamilton S . (1997): Olanzapine versus placebo and haloperidol: Acute phase results of the North American double-blind olanzapine trial. Neuropsychopharmacology 14: 111–123
Bell DS . (1965): A comparison of amphetamine psychosis and schizophrenia. Br J Psychiatry 3: 701–707
Braff DL, Geyer MA . (1990): Sensorimotor gating and schizophrenia: Human and animal model studies. Arch Gen Psychiatry 47: 181–188
Breier A, Adler CM, Weisenfeld N, Su TP, Elman I, Picken L, Malhotra AK, Pickar D . (1998): Effects of NMDA antagonism on striatal dopamine release in healthy subjects: Application of a novel PET approach. Synapse 29: 142–147
Breier A, Malhotra AK, Pinals DA, Weisenfeld NI, Pickar D . (1997): Association of ketamine-induced psychosis with focal activation of the prefrontal cortex in healthy volunteers. Am J Psychiatry 154: 805–811
Bymaster FP, Moore NA, Nakazawa T . (1999): Review of the preclinical pharmacology of olanzapine: A MARTA class antipsychotic. Psychopharmacology 2: 885–911
Carpenter WT Jr, Buchanan RW . (1994): Schizophrenia. N Engl J Med 330: 681–690
Carpenter WT Jr, Buchanan RW, Kirkpatrick B, Tamminga CA, Wood F . (1993): Strong inference, theory testing, and the neuroanatomy of schizophrenia. Arch Gen Psychiatry 50: 825–831
Casey DE . (1996): Behavioral effects of sertindole, risperidone, clozapine and haloperidol in Cebus monkeys. Psychopharmacology 124: 134–140
Cook L, Catania AC . (1964): Effects of drugs on avoidance and escape behavior. Fed Proc 23: 818–835
Corbett R, Camacho F, Woods AT, Kennan LL, Fishkin RJ, Brooks K, Dunn RW . (1995): Antipsychotic agents antagonize non-competitive N-methyl-D-aspartate antagonist-induced behaviors. Psychopharmacology 120: 67–74
Domino EF, Luby E . (1981): Abnormal mental states induced by phencyclidine as a model of schizophrenia. In Domino EF (ed), PCP (Phencyclidine): Historical and Current Perspectives. Ann Arbor, MI, NPP Books, pp 401–413
Duncan E, Madonick SH, Angrist BM, Bartlett E, Parwani A, Rotrosen JP . (1997): N-methyl-d- aspartate modulation of prepulse inhibition. Am Psychiatric Assoc Annual Meeting.
Duncan GE, Leipzig JN, Mailman RB, Lieberman JA . (1998): Differential effects of clozapine and haloperidol on ketamine-induced brain metabolic activation. Brain Res 812: 65–75
Ellenbroek B, Cools AR . (1988): The Paw test: An animal model for neuroleptic drugs which fulfills the criteria for pharmacological isomorphism. Life Sci 42: 1205–1213
Farber NB, Hanslick J, Kirby C, McWilliams L, Olney JW . (1998): Serotonergic agents that activate 5HT2A receptors prevent NMDA antagonist neurotoxicity. Neuropsychopharmacology 18: 57–62
Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC . (1994): Assessing the significance of focal activations using their spatial extent. Hum Brain Mapping 1: 210–220
Gao XM, Hashimoto T, Tamminga CA . (1998): Phencyclidine (PCP) and dizocilpine (MK801) exert time-dependent effects on the expression of immediate early genes in rat brain. Synapse 29: 14–28
Gao XM, Sakai K, Roberts RC, Conley RR, Dean B, Tamminga CA . (1999): Ionotropic glutamate receptors and NMDA subunit expression in subregions of human hippocampus: Effects of schizophrenia. Am J Psychiatry, in press.
Grace AA, Bunney BS, Moore H, Todd CL . (1997): Dopamine-cell depolarization block as a model for the therapeutic actions of antipsychotic drugs. TINS 20: 31–37
Griffith JD, Cavanaugh J, Held J . (1972): Dextroamphetamine: Evaluation of psychomimetic properties in man. Arch Gen Psychiatry 26: 97–100
Grotta J, Clark W, Coull B, Pettigrew LC, Mackay B, Goldstein LB, Meissner I, Murphy D, LaRue L . (1995): Safety and tolerability of the glutamate antagonist CGS 19755 (Selfotel) in patients with acute ischemic stroke. Results of a phase IIa randomized trial. Stroke 26: 602–605
Harborne GC, Watson FL, Healy DT, Groves L . (1996): The effects of sub-anaesthetic doses of ketamine on memory, cognitive performance and subjective experience in healthy volunteers. J Psychopharmacol 10: 134–140
Javitt DC, Zukin SR . (1991): Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 148: 1301–1308
Jentsch JID, Roth RH . (1999): The neuropsychopharmacology of phencyclidine: From NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology 20: 201–225
Kehne JH, Baron BM, Carr AA, Chaney SF, Elands J, Feldman DJ, Frank RA, VanGiersbergen PLM, McCloskey TC, Johnson MP, McCarty DR, Poirot M, Senyah Y, Siegel BW, Widmaier C . (1996): Preclinical characterization of the potential of the putative atypical antipsychotic MDL 100,907 as a potent 5-HT2A antagonist with a favorable CNS safety profile. J Pharmacol Exp Ther 277: 968–981
Kirkpatrick B, Buchanan R . (1990): The neural basis of the deficit syndrome of schizophrenia. J Nerv Ment Dis 178: 545–555
Kristensen JD, Svensson B, Gordh T Jr . (1992): The NMDA-receptor antagonist CPP abolishes neurogenic ‘wind-up pain’ after intrathecal administration in humans. Pain 51: 249–253
Kroeze WK, Roth BL . (1998): The molecular biology of serotonin receptors: Therapeutic implications for the interface of mood and psychosis. Biol Psychiatry 44: 1128–1142
Krystal JH, Karper LP, Bennett A, D'Souza DC, Abi-Dargham A, Morrissey K, Abi-Saab D, Bremmer JD, Bowers MB, Suckow RF, Stetson P, Heninger GR, Charney DS . (1998): Interactive effects of subanesthetic ketamine and subhypnotic lorazepam in humans. Psychopharmacology 135: 213–229
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremmer JD, Heninger GR, Bowers IMB, Charney DS . (1994): Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Arch Gen Psychiatry 51: 199–214
Lahti AC, Holcomb HH, Medoff DR, Tamminga CA . (1995a): Ketamine activates psychosis and alters limbic blood flow in schizophrenia. Neuroreport 6: 869–872
Lahti AC, Koffel B, LaPorte D, Tamminga CA . (1995b): Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology 13: 9–19
Lahti AC, Lahti RA, Tamminga CA . (1996): New neurolepties and experimental antipsychotics: Future roles. In Breier A (ed), The New Pharmacotherapy of Schizophrenia. Washington, DC, American Psychiatric Press, pp 57–87
Lahti AC, Holcomb HH, Weiler MA, Corey PK, Zhao M, Medoff D, Tamminga CA . (1997): Effects of ketamine on behavior and rCBF in schizophrenia and normal individuals. Biol Psychiatry 41: 165–169
Lahti AC, Weiler MA, Corey PK, Lahti RA, Carlsson A, Tamminga CA . (1998): Antipsychotic properties of the partial dopamine agonist (-)-3-(3-Hydroxyphenyl)-N-n-Propylpiperidine (Preclamol) in schizophrenia. Biol Psychiatry 43: 2–11
Lahti AC, Weiler MA, Parwani A, Holcomb HH, Michaelidis T, Warfel D, Tamminga CA . (1999): Blockade of ketamine-induced psychosis with olanzapine. Schizophr Res 36: 310
LaPorte DJ, Lahti AC, Koffel B, Tamminga CA . (1996): Absence of ketamine effects on memory and other cognitive functions in schizophrenic patients. J Psychiatr Res 30: 321–330
Leysen JE, Janssen PM, Megens AA, Schotte A . (1994): Risperidone: A novel antipsychotic with balanced serotonin-dopamine antagonism, receptor occupancy profile, and pharmacologic activity. J Clin Psychiatry 55: 5–12
Liddle PF, Friston KJ, Frith CD, Hirsch SR, Jones T, Frackowiak RS . (1992): Patterns of cerebral blood flow in schizophrenia. Br J Psychiatry 160: 179–186
Luby ED, Cohen BD, Rosenbaum G, Gottlieb JS, Kelley R . (1959): Study of a new schizophrenomimetic drug: serenyl. Arch Neurol Psychiatry 71: 363–369
Malhotra AK, Adler CM, Kennison SD, Elman 1, Pickar D, Breier A . (1997a): Clozapine blunts N-methyl-D-Aspartate antagonist-induced psychosis: A study with ketamine. Biol Psychiatry 42: 664–668
Malhotra AK, Pinals DA, Adler CM, Elman I, Clifton A, Pickar D, Breier A . (1997b): Ketamine-free schizophrenics. Neuropsychopharmacology 17: 141–150
Malhotra AK, Pinals DA, Weingartner H, Sirocco K, Missar CD, Pickar D, Breier A . (1996): NMDA receptor function and human cognition: The effects of ketamine in healthy volunteers. Neuropsychopharmacology 14: 301–307
Marder SR, Chouinard G, Jones B, Remington G, Bloom D, Addington D, MacEwan GW, Labelle A, Beauclair L, Arnott W . (1993): Risperidone: Clinical development: North American results. A Canadian multicenter placebo-controlled study of fixed doses of risperidone and haloperidol in the treatment of chronic schizophrenic patients. Clin Neuropharmacol 13: 25–40
McKinney WT, Bunney WE Jr . (1969): Animal model of depression. I. Review of evidence: Implications for research. Arch Gen Psychiatry 21: 240–248
Moghaddam B, Adams BW . (1998): Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science 281: 1349–1352
Moghaddam B, Adams BW, Verma A, Daly D . (1997): Activation of glutamatergic neurotransmission by ketamine: A novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 17: 2921–2927
Newcomer JW, Farber NB, Jevtovic-Todorovic V, Selke G, Melson AK, Hershey T, Craft S, Olney JW . (1999a): Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology 20: 106–118
Newcomer JW, Farber NB, Selke G, Melson AK, Jevtovic-Todorovic V, Olney JW . (1999b): Guanabenz prevention of NMDA antagonist-induced mental symptoms in healthy humans. Schizophr Res 36: 311
Overall JE, Gorham DR . (1962): The Brief Psychiatric Rating Scale. Psychol Rep 10: 799–812
Pearlson GD . (1981): Psychiatric and medical syndromes associated with phencyclidine (PCP) abuse. John Hopkins Med J 148: 25–33
Radant AD, Bowdle TA, Cowley DS, Kharasch ED, Roy-Byme PP . (1998): Does ketamine-mediated N-methyl-D-aspartate receptor antagonism cause schizophrenia-like oculomotor abnormalities? Neuropsychopharmacology 19: 434–444
Robertson GS, Matsumura H, Fibiger HC . (1994): Induction patterns of fos-like immunoreactivity in the forebrain as predictors of atypical antipsychotic activity. J Pharmacol Exp Ther 271: 1058–1066
Sams-Dodd F . (1999): Phencyclidine in the social interaction test: An animal model of schizophrenia with face and predictive validity. Rev Neurosci 10: 59–90
Sanger DJ . (1985): The effects of clozapine on shuttle-box avoidance responding in rats: Comparisons with haloperidol and chlordiazepoxide. Pharmacol Biochem Behav 23: 231–236
Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, De Loore K, Leysen JE . (1996): Risperidone compared with new and reference antipsychotic drugs: In vitro and in vivo receptor binding. Psychopharmacology 124: 57–73
Swerdlow NR, Bakshi V, Waikar M, Taaid N, Geyer MA . (1998): Seroquel, clozapine and chlorpromazine restore sensorimotor gating in ketamine-treated rats. Psychopharmacology 140: 75–80
Tamminga CA, Dale JM, Goodman L, Kaneda H, Kaneda N . (1990): Neuroleptic-induced vacuous chewing movements as an animal model of tardive dyskinesia: a study in three rat strains. Psychopharmacology 102: 474–478
Tamminga CA, Schaffer MH . (1979): Treatment of schizophrenia with ergot derivatives. Psychopharmacology 66: 239–242
Tamminga CA, Tanimoto K, Kuo S, Chase TN, Contreras PC, Rice KC, Jackson AE, O'Donohue TL . (1987): PCP-induced alterations in cerebral glucose utilization in rat brain: Blockade by metaphit, a PCP-receptor-acylating agent. Synapse 1: 497–504
Vollenweider EX, Leenders KL, Scharfetter C, Antonini A, Maguire P, Missimer J, Angst J . (1997): Metabolic hyperfrontality and psychopathology in the ketamine model of psychosis using positron emission tomography (PET) and [18F]fluorodeoxyglucose (FDG). Eur Neuropsychopharmacol 7: 9–24
Vollenweider FX, Leenders KL . (1999): A role for cortico-striato-thalamic loops in model psychoses: A PET study with FDG. Schizophr Res 36: 236
Waddington JL . (1990): Spontaneous orofacial movements induced in rodents by very long-term neuroleptic drug administration: Phenomenology, pathophysiology and putative relationship to tardive dyskinesia. Psychopharmacology 101: 431–447
Weiler MA, Thaker GK, Lahti AC, Ross DE, Tamminga CA . (1997): Effects of ketamine on eye tracking in normal controls. Schizophr Res 24: 246
Weinberger DR . (1995): Neurodevelopmental perspectives on schizophrenia. In Anonymous, Psychopharmacology: The Fourth Generation of Progress. New York, Raven Press, Ltd, pp 1171–1183
Weiss JM, Kilts CD . (1995): Animal models of depression and schizophrenia. In Schatzberg AF, Nemeroff CV (eds), Textbook of Psychopharmacology. Washington, DC, American Psychiatric Press, pp 81–123
Weissman AD, Dam M, London EG . (1987): Alterations in local cerebral glucose utilization induced by phencyclidine. Brain Res 435: 29
Worms P, Broekkamp CLE, Lloyd KG . (1983): Behavioral effects of neuroleptics. In Coyle JT, Enna SJ (eds), Neuroleptics: Neurochemical, Behavioral, and Clinical Perspectives. New York, Raven, pp 93–117
Zimbroff DL, Kane JM, Tamminga CA, Daniel DG, Mack RJ, Womiak PJ, Sebree TB, Wallin BA, Kashkin KB . (1997): A controlled, dose-response study of sertindole and haloperidol in schizophrenia. Am J Psychiatry 154: 782–791
Zukin SR, Fitz-Syage L, Nichtenhauser R, Zukin RS . (1983): Specific binding of [3H]phencyclidine in rat central nervous tissue: Further characterization and technical considerations. Brain Res 258: 277–284
Zukin SR, Zukin RS . (1979): Specific [3H]phencyclidine binding in rat central nervous system. Proc Natl Acad Sci USA 76: 5372–5376
Acknowledgements
Supported by an unrestricted educational grant from Hoechst Marion Roussel.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lahti, A., Holcomb, H., Gao, XM. et al. NMDA-Sensitive Glutamate Antagonism: A Human Model for Psychosis. Neuropsychopharmacol 21 (Suppl 2), S158–S169 (1999). https://doi.org/10.1016/S0893-133X(99)00132-3
Received:
Revised:
Accepted:
Issue date:
DOI: https://doi.org/10.1016/S0893-133X(99)00132-3
Keywords
This article is cited by
-
Effects of quetiapine and sertindole on subchronic ketamine-induced deficits in attentional set-shifting in rats
Psychopharmacology (2012)
-
Psychiatric safety of ketamine in psychopharmacology research
Psychopharmacology (2007)
-
Correlations Between rCBF and Symptoms in Two Independent Cohorts of Drug-Free Patients with Schizophrenia
Neuropsychopharmacology (2006)