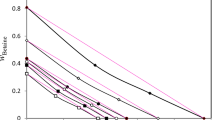
Abstract
WITHIN recent years considerable doubt has been cast upon the validity of the Gibbs adsorption isotherm as applied to aqueous solutions of the paraffin–chain salts (soaps and soap–like molecules)1,2. The chief objection has been, the numerous well–established examples of dilute solutions showing a minimum in the surface tension – concentration curve, usually at a surface tension of 30–35 dynes, presenting the paradox of a surface tension very much lower than that of water, and yet a zero or negative surface excess of solute as calculated from the Gibbs equation when applied in the customary manner.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
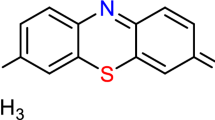
References
McBain and Mills, "Reports on Progress in Physics", 5, 30 (1939).
McBain and Wood, Proc. Roy. Soc., A, 174, 286 (1940).
Long and Nutting, J. Amer. Chem. Soc., 63, 625 (1941).
Powney and Addison, Trans. Faraday Soc., 33, 1252 (1937).
Lawrence, Annual Reports Chem. Soc., 37, 102 (1941).
Murray, Trans. Faraday Soc., 31, 206 (1935); Alexander, Trans. Faraday Soc., 37, 15 (1941).
cf. Nickerson, J. Phys. Chem., 40, 285 (1936).
Bury and others, Phil. Mag., 4, 841 (1927); J. Chem. Soc., 679 (1929); Hartley, "Aqueous Solutions of Paraffin-Chain Salts" (Hermann, 1936).
Alexander, in the press.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
ALEXANDER, A. Application of the Gibbs Adsorption Equation to Solutions of Paraffin–Chain Salts. Nature 148, 752 (1941). https://doi.org/10.1038/148752a0
Issue date:
DOI: https://doi.org/10.1038/148752a0