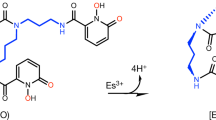
Abstract
IN view of the recent discovery of elements with atomic numbers 93–96, it is of interest to recall a calculation based on the assumption of a minimum proper length for the world-line of an electron1. The conception that it is impossible to discriminate in space-time between two positions of the electron when the interval separating them is less than h/m0c (m0 = rest mass) leads to the result that an electron in a Bohr orbit cannot have a velocity greater than c/2. A further consequence of this is that a K-ring cannot exist in an atom for which Z> hc/22pie2and this means that the Bohr-Rutherford model would stop at Z = 96. Although the original argument leading to this result cannot be regarded as free from objection in the light of modern quantum theory, the likelihood of some such limitation remains. A more recent application of the principle of minimum length and time to the Rutherford-Bohr atom sets the upper limit to Z somewhat lower than 962.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Fisher, J. W. and Flint, H. T., Proc. Roy. Soc., A, 115, 210 (1927). Flint, H. T., and Richardson, O. W., Proc. Roy. Soc., A, 117, 637 (1928). Flint, H. T., Proc. Roy. Soc., A, 117, 630 (1928).
Glaser, W., and Sitte, K., Z. Phys., 87, 674 (1934).
Bohr, N., and Wheeler, J. A., Phys. Rev., 56, 426 (1939).
Nature, 159, 243 (1947).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
FISHER, J., FLINT, H. X-Ray Spectra of Trans-Uranic Elements. Nature 159, 741 (1947). https://doi.org/10.1038/159741a0
Issue date:
DOI: https://doi.org/10.1038/159741a0