Abstract
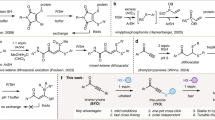
IT was first shown by Ziegler and co-workers1 that N-bromosuccinimide (XNBr) reacts with cyclohexene to give 3-bromocyclohexene (I) and succinimide. We have found that if the reaction is carried out in chloroform, benzene or petrol solution in the presence of salts such as tetraethylammonium bromide, tetraethylammonium iodide, or triethylamine hydrobromide, 1: 2-dibromocyclohexane (II) is formed instead of 3-bromocycloohexene, in proportions depending on the exact conditions employed. The same effect can be brought about by the addition of small amounts (c. 10 mol. per cent based on N-bromosuccinimide) of triethylamine, which reacts with part of the N-bromosuccinimide to give triethylamine hydrobromide and succinimide.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Ziegler, Späth, Schaaf, Schumann and Winkelmann, Annalen, 551, 80 (1942).
Howton, J. Amer. Chem. Soc., 69, 2060 (1947). Buchmann and Howton, ibid., 70, 2517, 3510 (1948).
Buckles, J. Amer. Chem. Soc., 71, 1157 (1949).
Bloomfleld, J. Chem. Soc., 114 (1944).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BRAUDE, E., WAIGHT, E. N-Bromosuccinimide as a Reagent for Bromine Addition. Nature 164, 241 (1949). https://doi.org/10.1038/164241a0
Issue date:
DOI: https://doi.org/10.1038/164241a0