Abstract
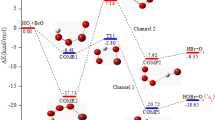
SIR CYRIL HINSHELWOOD has recently1 revived the old Noyes—Lowry suggestion that bromine may sometimes react in water after complete dissociation, Br2 ⇌ Br- + Br+, and not merely as an inductively polarized molecule, while Weiss2 has tentatively suggested that the simple bromine cation may exist in acid solutions of hypobromous acid: HOBr + H+ ⇌ Br+ + H2O. Direct experimental support for the latter suggestion has not as yet been put forward.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Hinshelwood, C. N., J. Chem. Soc., 696 (1947).
Weiss, J., Chemical Society, Ann. Reports, 44, 79 (1947).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
DERBYSHIRE, D., WATERS, W. The Bromine Cation, Br+, and its Reactions. Nature 164, 446–447 (1949). https://doi.org/10.1038/164446a0
Issue date:
DOI: https://doi.org/10.1038/164446a0
This article is cited by
-
An Oxidation Involving the Hydroxyl Cation, (OH)+
Nature (1950)
-
Positive Bromine Ions
Nature (1950)