Abstract
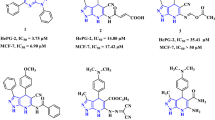
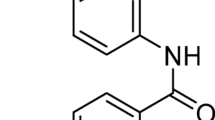
THE ease with which 1-substituted pyrazoles may be converted into the free imino compounds varies with the nature of the 1-substituent and the reacting substance. Thus, 1,3,5-trimethyl pyrazole may be nitrated1 and 1-phenyl pyrazole reduced to the pyrazoline2 without loss of the N-methyl or N-phenyl groups respectively. On the other hand, pyrazoles of the 1-carbamyl or 1-phenylcarbamyl type, (I), derived from the acyl hydrazides, semicarbazide3 and 4-phenylsemicarbazide4, are much less stable and readily give the free imino derivatives. 
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Knorr, Ann., 279, 234 (1894).
Balbiano, Gazz. chim. ital., 18, 358 (1888).
Posner, Ber., 34, 3979 (1901). von Auwers, Ber., 58, 2075 (1925). Dornlow and Peterlein, Ber., 82, 257 (1949).
Wheeler and Norton, J. Amer. Chem. Soc., 50, 2488 (1928).
Thiele and Bihan, Ann., 302, 299 (1898). Thiele and Strange, Ann., 283, 27 (1894). De, Quart J. Ind. Chem. Soc., 4, 183 (1927).
Hantzsch and Vagt, Ann., 314, 339 (1906).
Thiele and Dralle, Ann., 302, 275 (1898). See also De and Rakshit, J. Ind. Chem. Soc., 13, 509 (1936).
O'Connor, Horgan and Reilly, J. App. Chem., 1, 91 (1951).
Scott, F. L., and O'Sullivan, D. A. (unpublished work).
Musante, Gazz. chim. ital., 72, 537 (1942).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
SCOTT, F., MURPHY, C. & REILLY, J. Carbamidine-substituted Pyrazoles. Nature 167, 1037 (1951). https://doi.org/10.1038/1671037a0
Issue date:
DOI: https://doi.org/10.1038/1671037a0
This article is cited by
-
A New Route to Substituted Guanidines
Nature (1952)