Abstract
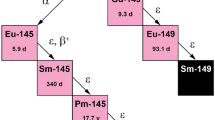
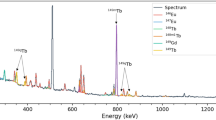
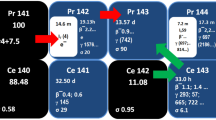
CONSIDERATION of nuclear systematics shows that, if any stable isotope of promethium exists, it would almost certainly have a mass number 145, 147 or 149, or possibly 143 with the stable 82-neutron configuration. It is, however, already known1,2 that the isotopes 147, 149 and 143 have half-lives of about four years, 47 hr. and 350 days respectively. It is therefore of interest to investigate whether the unknown promethium-145 is radioactive.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Seaborg, G. T., and Perlman, I., “Table of Isotopes”, Rev. Mod. Phys., 20, 585 (1948).
Hicks, H. G., A.E.C.U., 217.
Inghram, M. G., Hayden, R. J., and Hess, D. C., Phys. Rev., 71, 643 (1947).
Ketelle, B. H., and Boyd, G. E., J. Amer. Chem. Soc., 69, 2800 (1947).
Cork, J. M., Shreffler, R. G., and Fowler, C. M., Phys. Rev., 74, 240 (1948).
Scharff-Goldhaber, G., der Mateosian, E., McKeown, M., and Sunyar, A. W., A.E.C.U., 767.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BUTEMENT, F. Radioactive Samarium-145 and Promethium-145. Nature 167, 400 (1951). https://doi.org/10.1038/167400a0
Issue date:
DOI: https://doi.org/10.1038/167400a0