Abstract
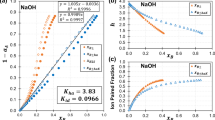
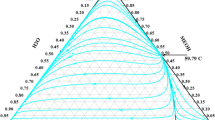
IN a recent paper1, A. C. Walker has given tables and curves for the solubility–temperature relationship in the system ethylenediamine D-tartrate (anhyd.)–ethylenediamine D-tartrate monohydrate–water. Dr. Walker shows that the salt decomposes slowly in solution, and the consequent change of composition of the solute during the time taken by the solubility determinations introduces some uncertainty in the results. Usually, when preparing a solubility curve, a considerable time must be allowed, at each observed temperature, for equilibrium between solution and solute to be attained. The change of composition of the solute during the experiments is probably responsible for the difference in level, of the order of 1° C., in two of the published curves.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Walker, A. C., J. Franklin Inst., 250, 481 (1950).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
DAUNCEY, L., STILL, J. Solubility of Ethylenediamine D-Tartrate in Water. Nature 168, 34 (1951). https://doi.org/10.1038/168034a0
Issue date:
DOI: https://doi.org/10.1038/168034a0