Abstract
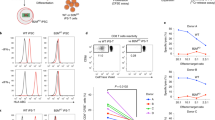
We present a clinical scale method for immunomagnetic separation of CD56+ donor natural killer cells for adoptive immunotherapy of pediatric leukemias after allogeneic transplantation. This time-saving and partially automated procedure employed CD56+ selection followed by CD3+ depletion, resulting in a median purity of 98.6% NK cells and a four-log depletion of T cells. The enriched NK cells demonstrated high cytotoxic activity against K562 target cells and fresh leukemic blasts with low HLA class I expression, which could be further enhanced by IL-2 stimulation. Lysis of NK-insensitive leukemic cells with high HLA class I expression could also be demonstrated via ADCC. Due to the high degree of T cell depletion, alloreactive proliferation in mixed lymphocyte cultures and response to T cell-specific mitogen stimulation was profoundly decreased. Our results suggest that, even in the case of mismatched donors, infusions of donor NK cells with extremely low T cell content may be a promising treatment option for leukemic minimal residual disease after allogeneic transplantation without risk of inducing severe GVHD.
Bone Marrow Transplantation (2002) 29, 497–502. doi:10.1038/sj.bmt.1703406
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Handgretinger R, Schumm M, Lang P et al. Transplantation of megadoses of purified haploidentical stem cells Ann N Y Acad Sci 1999 872: 350 362
Handgretinger R, Klingebiel T, Lang P et al. Megadose transplantation of purified peripheral blood CD34+ progenitor cells from HLA-mismatched parental donors in children Bone Marrow Transplant 2001 27: 777 783
Aversa R, Tabilio A, Velardi A et al. Treatment of high-risk acute leukemia with T cell depleted stem cells from related donors with one fully mismatched HLA haplotype New Engl J Med 1998 339: 1186 1193
Verdonck LF, Petersen EJ, Lokhorst HM et al. Donor leukocyte infusions for recurrent hematological malignancies after allogeneic bone marrow transplantation: impact of infused and residual donor T cells Bone Marrow Transplant 1998 22: 1057 1063
Klein JP, Keiding N, Shu Y et al. Summary curves for patients transplanted for chronic myeloid leukaemia salvaged by a donor lymphocyte infusion: the current leukaemia-free survival curve Br J Haematol 2000 109: 148 152
Bader P, Klingebiel T, Schaudt A et al. Prevention of relapse in pediatric patients with acute leukemias and MDS after allogeneic SCT by early immunotherapy initiated on the basis of increasing mixed chimerism: a single center experience of 12 children Leukemia 1999 13: 2079 2086
Collins RH, Goldstein S, Giralt S et al. Donor leukocyte infusions in acute lymphocytic leukemia Bone Marrow Transplant 2000 26: 511 516
Horowitz MM, Gale RP, Sondel PM et al. Graft-versus-leukemia reactions after bone marrow transplantation Blood 1990 75: 555 562
Porter DL, Collins RH, Hardy C et al. Treatment of relapsed leukemia after unrelated donor marrow transplantation with unrelated donor leukocyte infusion Blood 2000 95: 1214 1221
Ljunggren HB, Kärre K . In search of the ‘missing self’: MHC molecules and NK cell recognition Immunol Today 1990 11: 237 244
Reyburn H, Mandelboim O, Valés-Goméz M et al. Human NK cells: their ligands, receptors and functions Immunol Rev 1997 155: 119 125
Ruggeri L, Capanni M, Casucci M et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation Blood 1999 94: 334 339
Lister J, Rybka WB, Donnenberg AD et al. Autologous peripheral blood stem cell transplantation and adoptive immunotherapy with A–NK cells in the immediate post-transplant period Clin Cancer Res 1995 1: 607 614
Rosenberg SA, Lotze MT, Muul LM . A progress report on the treatment of 157 patients with advanced cancer using lymphokine activated killer cells and interleukin-2 or high dose interleukin-2 alone New Engl J Med 1987 316: 889 897
Benyunes M, Higuchi C, York A et al. Immunotherapy with interleukin-2 with or without lymphokine-activated killer cells after autologous bone marrow transplantation for malignant lymphoma: a feasibility trial Bone Marrow Transplant 1995 16: 283 288
Zeis M, Uharek L, Glass B et al. Induction of graft-versus-leukemia activity in murine leukemia models after IL-2 pretreatment of syngeneic and allogeneic bone marrow grafts Bone Marrow Transplant 1994 14: 711 715
Asai O, Longo D, Tian Z et al. Suppression of graft-versus-host disease and amplification of graft-versus-tumor effects by activated natural killer cells after allogeneic bone marrow transplantation J Clin Invest 1998 101: 1835 1842
Kimoro Y, Tanaka T, Tanji Y et al. Use of human leukocyte antigen-mismatched allogeneic lymphokine-activated killer cells and interleukin-2 in the adoptive immunotherapy of patients with malignancies Biotherapy 1995 8: 41 50
Boughton B, Simpson A, Phaure T et al. Graft-versus-host-disease following interleukin-2/lymphokine-activated killer (LAK) cell immunotherapy in a patient with acute myelogenous leukaemia in second complete remission: autologous LAK cells following allogeneic bone marrow transplantation are donor-derived Cancer Immunol Immunother 1995 41: 68 70
Geiselhart A, Neu S, Buchholz F et al. Positive selection of CD56+ lymphocytes by magnetic cell sorting Nat Immunol 1996–97 15: 227 233
Murphy WJ, Keller JR, Harrison CL et al. Interleukin 2 activated natural killer cells can support hematopoiesis in vitro and promote marrow engraftment in vivo Blood 1992 80: 670 677
Acknowledgements
We thank Shangara Lal for critical reviewing of the manuscript and Ulrike Krauter for excellent technical assistance. This work was supported by a grant from the German Jose Carreras Leukemia Foundation and from the Deutsche Forschungs-Gesellschaft (SFB 510).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lang, P., Pfeiffer, M., Handgretinger, R. et al. Clinical scale isolation of T cell-depleted CD56+ donor lymphocytes in children. Bone Marrow Transplant 29, 497–502 (2002). https://doi.org/10.1038/sj.bmt.1703406
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.bmt.1703406
Keywords
This article is cited by
-
The role of NK cells in HIV-1 protection: autologous, allogeneic or both?
AIDS Research and Therapy (2016)
-
Reduction of Minimal Residual Disease in Pediatric B-lineage Acute Lymphoblastic Leukemia by an Fc-optimized CD19 Antibody
Molecular Therapy (2016)
-
IL-15-stimulated CD3/CD19-depleted stem-cell boosts in relapsed pediatric patients after haploidentical SCT
Leukemia (2012)
-
Tumour stromal cells derived from paediatric malignancies display MSC-like properties and impair NK cell cytotoxicity
BMC Cancer (2010)
-
Natürliche Killerzellen in der Leukämie- und Tumortherapie
Monatsschrift Kinderheilkunde (2010)