Abstract
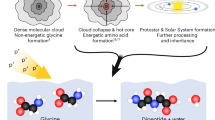
INTEREST in the crystal structures of simple peptides has increased considerably during recent years on account of progress made in the direct investigation of protein structure. Glycyl-L-alanine hydrobromide has been prepared in these laboratories and examined by X-ray methods. The free dipeptide was prepared by the N-carbobenzoxy method1 and then converted into the hydrobromide which crystallized readily from aqueous solution.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Bergmann, M., and Zervas, L., Ber. Dtsch. Chem. Ges., 65, 1192 (1932).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
TRANTER, T. Crystal Structure of Glycyl-L-Alanine Hydrobromide. Nature 173, 221–222 (1954). https://doi.org/10.1038/173221a0
Issue date:
DOI: https://doi.org/10.1038/173221a0
This article is cited by
-
Comments on “Crystal growth, spectral, optical, and thermal characterization of glycyl-l-alanine hydrochloride (GLAH) single crystal”
Journal of Thermal Analysis and Calorimetry (2014)
-
Crystal Structure of Glycyl-L-Alanine Hydrochloride
Nature (1956)