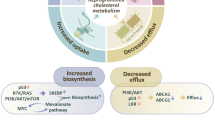
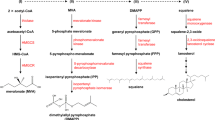
Abstract
IN the course of more extensive work on degradation of cholesterol, we have made a stereochemical correlation which seems to justify an interim report. 
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Cf. Achtermann, T., Z. physiol. Chem., 225, 141 (1934). Laucht, F., Z. physiol. Chem., 237, 236 (1935), for analogous work with ergosterol.
Carlisle, C. H., and Crowfoot, D., Proc. Roy. Soc., A, 184, 64 (1945).
Cf. Klyne, W., Chem. and Indust., 426 (1951).
Cf. Birch, A. J., Ann. Reports Chem. Soc., 47, 192 (1950). Inter-mediate stages are isopulegol, pulegone and β-methyladipic acid.
Fredga, A., and Leskinen, E., Svedberg Memorial Volume, 261 (1944); Ark. Kemi, Min. Geol., 19 B, No. 1 (1944).
Mills, J. A., J. Chem. Soc., 4976 (1952); Chem. and Indust., 218 (1953).
Prelog, V., Helv. Chim. Acta, 36, 308 (1953). Prelog, V., et al., Helv. Chim. Acta, 36, 320, 325 and 1178 (1953).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
CORNFORTH, J., YOUHOTSKY, I. & POPJÁK, G. Absolute Configuration of Cholesterol. Nature 173, 536 (1954). https://doi.org/10.1038/173536a0
Issue date:
DOI: https://doi.org/10.1038/173536a0
This article is cited by
-
Transformed steroids
Bulletin of the Academy of Sciences of the USSR Division of Chemical Science (1971)