Abstract
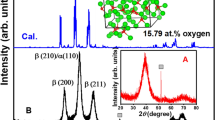
PREVIOUS work1 has shown that hydrogen reduction of tungsten trioxide gave the β-oxide (W20O58)2, which was then apparently reduced both to β-tungsten (a = 5.04 A.) and to tungsten dioxide or the γ-oxide.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Charlton, M. G., Nature, 169, 109 (1952).
Magnéli, A., Ark. Kem., 1, 513 (1950).
Magnéli, A., Ark. Kem., 1, 223 (1949).
Hägg, G., and Schönberg, N., Acta Cryst., 7, 351 (1954).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
CHARLTON, M. Hydrogen Reduction of Tungsten Oxides. Nature 174, 703 (1954). https://doi.org/10.1038/174703a0
Issue date:
DOI: https://doi.org/10.1038/174703a0
This article is cited by
-
Metals Production and Metal Oxides Reduction Using Hydrogen: A Review
Journal of Sustainable Metallurgy (2022)
-
In situ TEM observations of irradiation-induced phase change in tungsten
Journal of Materials Science (2009)
-
The preparation and characterization of beta-tungsten, a metastable tungsten phase
Metallurgical transactions (1974)