Abstract
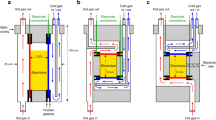
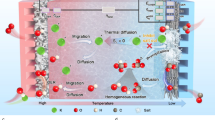
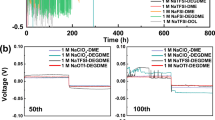
IN a recent communication1, Tyrrell and Colledge have directed attention to the possibility of studying the Soret effect in solutions of electrolytes by observations of the e.m.f. of non-isothermal cells. In such cells, two reversible electrodes are maintained at different temperatures; owing to thermal diffusion, the concentration of solute around one electrode increases and that around the other decreases. In consequence, an additional e.m.f. should gradually be set up, which, for small temperature differences, is simply the e.m.f. of an isothermal concentration cell (with transport) operating at the mean temperature.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Tyrrell, H. J. V., and Colledge, R., Nature, 173, 264 (1954).
Stokes, R. H., J. Amer. Chem. Soc., 72, 763 (1950).
Haase, R., Trans. Farad. Soc., 49, 724 (1953). Khoroshin, A. V., and Temkin, M. I., Zhur. Fiz. Khim., 26, 773 (1952).
de Groot, S. R., “L'Effet Soret” (Amsterdam, 1945).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
AGAR, J., BRECK, W. Thermal Diffusion Potentials and the Soret Effect. Nature 175, 298–299 (1955). https://doi.org/10.1038/175298b0
Issue date:
DOI: https://doi.org/10.1038/175298b0