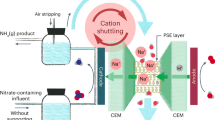
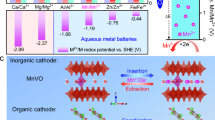
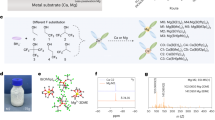
Abstract
WHEN nitrate solutions are employed as the catholyte in an electrolytic cell, with the electrodes separated by an anion-permeable ion-exchange membrane, many metallic hydroxides tend to precipitate in the cathode compartment, to compensate for the loss of nitrate ions to the anolyte. For a trivalent cation, the overall reaction can be represented as follows: 
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
See, for example, Brit. Pat. 640,438. Booth, H. S., “Inorganic Syntheses”, 1, 186 (McGraw-Hill, New York, 1939).
Braun, P. B., Nature, 170, 1123 (1952).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
DAVEY, P., SCOTT, T. Preparation of Maghæmite by Electrolysis. Nature 179, 1363 (1957). https://doi.org/10.1038/1791363a0
Issue date:
DOI: https://doi.org/10.1038/1791363a0
This article is cited by
-
The observation of enantiomorphous domains in a natural maghemite
Contributions to Mineralogy and Petrology (1979)
-
Oriented Transformations in Iron Oxides and Hydroxides
Nature (1957)