Abstract
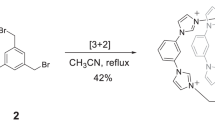
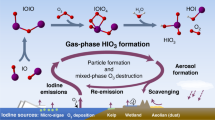
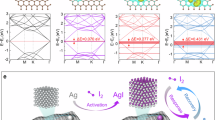
WHEN the pH of an I3 − solution, approximately one-tenth molar in iodide, is raised to about 12, part of the I3 − initially present disappears immediately, and the remainder decomposes slowly, at a measurable rate, due to production of iodate (Fig. 1).
Similar content being viewed by others
Article PDF
References
Herbo, C., and Sigalla, J., Anal. Chim. Acta, 17, 199 (1957).
Fürth, A., Z. Elektrochem., 28, 57 (1922).
Skrabal, A., Chem. Ber., 75, 1570 (1942).
Allen, T. L., and Keefer, R. M., J. Amer. Chem. Soc., 77, 2957 (1955).
Angelescu, E., and Popescu, V. D., Z. phys. Chem., A, 156, 304 (1931).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
SIGALLA, J. A New Iodine Compound: I−OH2 . Nature 183, 178 (1959). https://doi.org/10.1038/183178a0
Issue date:
DOI: https://doi.org/10.1038/183178a0