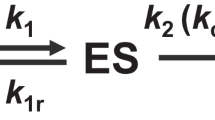Abstract
IN a recent paper1, Leo Szilard has shown that a system consisting of an enzyme, an enzyme-forming apparatus and a repressor may be able to exist in two stable states, provided the combining energies between the various components of this system lie within certain ranges of magnitude. The two stable states which can exist contain either high or low concentrations of the enzyme. If the concentration of the enzyme or the repressor is changed by external manipulation, the system returns to one or the other of the stable states. Such a system was termed ‘para-constitutive’ by Szilard, and its possible biological significance in differentiation and antibody formation2 was suggested by him.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Szilard, L., Proc. U.S. Nat. Acad. Sci., 46, 277 (1960).
Szilard, L., Proc. U.S. Nat. Acad. Sci., 46, 293 (1960).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
ANKER, H. A Possible Biochemical Mechanism for Memory. Nature 188, 938 (1960). https://doi.org/10.1038/188938a0
Issue date:
DOI: https://doi.org/10.1038/188938a0
This article is cited by
-
Evolution in Open Systems: Bistability and the Origin of Molecular Asymmetry
Nature New Biology (1973)
-
Bistability caused by Substrate Inhibition of Peroxidase in an Open Reaction System
Nature (1968)