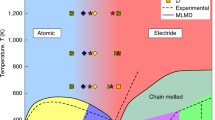
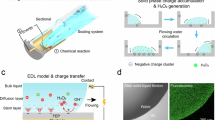
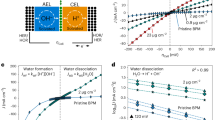
Abstract
IT is assumed in measuring potential with hydrogen or alkali-sensitive glass electrodes1 that the salt bridge which connects the reference electrode to the solution generates a negligibly small potential at the liquid junction. This is a reasonable assumption so long as the unknown solution contains no colloids, and its ionic strength is approximately that of the buffers used in standardization. Unfortunately these ideal conditions are seldom met in biological systems. The resultant error is often of a serious magnitude, as shown in experiments on pH in colloidal systems2,3.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Isard, J. O., Nature, 184, 1616 (1959).
Jenny, H., Neilson, T. R., Coleman, N. T., and Williams, D. E., Science, 119, 164 (1950).
Peech, M., Olsen, R. A., and Bolt, G. H., Soil Sci. Soc. Amer. Proc., 17.214 (1953).
Hitchcock, D. I., and Peters, R., J. Amer. Chem. Soc., 68, 1753 (1946).
Bates, R. G., Ann. New York Acad. Sci., 92, 341 (1961).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
MERRIL, C. Elimination of the Liquid Junction by using Glass Electrodes. Nature 192, 1087 (1961). https://doi.org/10.1038/1921087a0
Issue date:
DOI: https://doi.org/10.1038/1921087a0