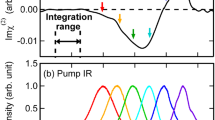
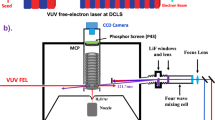
Abstract
MANY examples of changes in ultra-violet absorption spectra attributable to hydrogen bonding have been reported and summarized by Ito1. However, in few of these cases has the change in absorptivity been as pronounced as in the infra-red O–H stretching vibrations. The reason for this is probably due to the fact that the transitions responsible for the ultra-violet absorption have not been localized in that portion of the molecule most directly affected by the hydrogen bond. In this communication attention is directed to ultra-violet absorption bands of alcohols that vary drastically with concentration and probably arise from excitation of lone-pair electrons on an oxygen atom acting as a proton acceptor2.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Ito, M., J. Mol. Spect., 4, 125 (1960).
Mulliken, R. S., J. Chem. Phys., 3, 506 (1935).
Mecke, R., Disc. Farad. Soc., 9, 161 (1950).
Harrison, A. J., Cederholm, B. J., and Terwilliger, M. A., J. Chem. Phys., 30, 355 (1959).
Barrett, J., and Mansell, A. L., Nature, 187, 138 (1960).
Watanabe, K., and Zelikoff, M., J. Opt. Soc. Amer., 43, 753 (1953).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
KAYE, W., POULSON, R. Far Ultra-Violet Absorption Spectra of Hydrogen-bonded Methanol. Nature 193, 675–677 (1962). https://doi.org/10.1038/193675a0
Issue date:
DOI: https://doi.org/10.1038/193675a0