Abstract
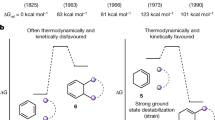
PREVIOUS work carried out at the Mass Spectrometry Centre of the University of Liège has shown the specific behaviour under electron impact of cis and trans isomers in a series of pairs of cyclanic stereoisomers. It has been especially pointed out in 1,2-dialkyl derivatives of cyclopropane1, cyclobutane2, cyclopentane3 and cyclohexane4 that in the ionized state the cis isomer is less stable than the corresponding trans isomer (that is, the relative abundance, expressed as a fraction of total ionic current for 70 V. energy electrons, of molecular ions of a cis isomer is smaller than that for the corresponding trans molecule).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
D'Or, L., Momigny, J., and Natalis, P., Symp. Mass Spectrometry (Oxford, September, 1961).
Laune, J., thesis, University of Liège (1961).
Natalis, P., Bull. Soc. Chim. Belg., 66, 5 (1957).
Natalis, P., Bull. Soc. Chim. Belg., 69, 519 (1960).
Amer. Petroleum Inst. Res. Project 44, Mass Spectral Data.
Beckett, C. W., Pitzer, K. S., and Spitzer, R., J. Amer. Chem. Soc., 69, 2488 (1947).
Johnson, W. H., Prosen, E. J., and Rossini, F. D., J. Res. Nat. Bur. Stand., 42, 251 (1949).
Pitzer, K. S., J. Chem. Phys., 8, 711 (1940).
Haresnape, J. N., Chem. and Indust., 1091 (1953).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
NATALIS, P. Assignment of cis–trans Configuration to 1,2-Dialkylcyclohexanes. Nature 195, 380 (1962). https://doi.org/10.1038/195380a0
Issue date:
DOI: https://doi.org/10.1038/195380a0