Abstract
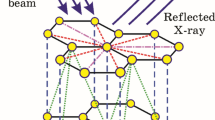
WHEN subjected to an atmosphere of bromine, graphite takes up bromine and swells, ultimately reaching a volume 55 per cent greater than its original volume1. The mode of adsorption is said to be by intercalation between the layers of graphite atoms. We have been able to obtain electron and X-ray spot diffraction patterns from relatively perfect natural single crystals of graphite which have been brominated previously for periods of 2–60 h.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Saunders, G. A., Ubbelohde, A. R., and Young, D. A., Proc. Roy. Soc., A. 271, 499 (1963).
Rudorff, W., Z. Anorg. Allg. Chem., 245, 383 (1941).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
EELES, W., TURNBULL, J. Electron and X-ray Diffraction applied to Brominated Graphite. Nature 198, 877–878 (1963). https://doi.org/10.1038/198877a0
Issue date:
DOI: https://doi.org/10.1038/198877a0