Abstract
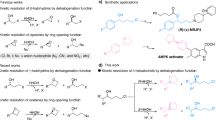
DURING experiments aimed at the microbiological side-chain degradation of cholesterol and cholestenone, reaction products were sometimes observed, which apparently were formed under the influence of sunlight in methylene chloride (Fig. 1). This solvent was used for extraction of the culture media. Like other halogenated hydrocarbons it is frequently utilized in microbiological preparation of steroids1 and in their biochemical, spectro-scopical and clinical analysis. The transformations described here may therefore be of general interest.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Eppstein, S. H., et al., Vitamins and Hormones, 14, 424 (1956).
Neher, R., and Wettstein, A., Helv. Chim. Acta, 35, 276 (1952). Loomeyer, F. J., J. Chromatog., 1, 179 (1958).
Ritter, F. J., and Hartel, J., Nature, 181, 765 (1958); J. Chromatog., 1, 461 (1958).
Dam, M. J. D. van, et al., J. Chromatog., 4, 26 (1960).
Ritter, F. J., and Meyer, G. M., Nature, 193, 941 (1962).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
RITTER, F. Light-induced Oxidation and Halogenation of Δ4-3-Keto-steroids in Halogenated Solvents. Nature 202, 694–695 (1964). https://doi.org/10.1038/202694a0
Issue date:
DOI: https://doi.org/10.1038/202694a0