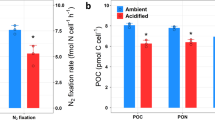
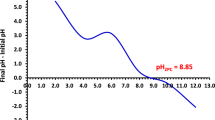
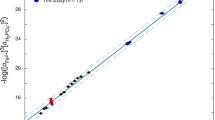
Abstract
DURING some experiments in which sodium dithionite, Na2S2O4, was employed as a reducing agent in aqueous phosphate buffer, it was noticed that the presence of condensed phosphates could be chromatographically detected at the end of each experiment. It was soon verified that sodium dithionite alone was responsible for this phenomenon.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Miller, S. L., and Parris, M., Nature, 204, 1248 (1964).
Steinman, G., Lemmon, R. M., and Calvin, M., Proc. US Nat. Acad. Sci., 52, 27 (1964).
Beck, A., and Orgel, L. E., Nature, 208, 1000 (1965).
Beck, A., and Orgel, L. E., Proc. US Nat. Acad. Sci., 54, 664 (1965).
Karl-Kroupa, E., Anal. Chem., 28, 1091 (1956).
Fuchs, R. J., and Czeck, F. W., Anal. Chem., 35, 769 (1963).
Chess, W. B., and Bernhart, D. N., Anal. Chem., 31, 1116 (1959).
Bartlett, G. R., J. Biol. Chem., 234, 466 (1959).
Latimer, W. M., The Oxidation States of the Elements and Their Potentials in Aqueous Solutions, second ed., 77 (Prentice–Hall, New Jersey, 1952).
Rittmann, A., Volcanoes and Their Activity (John Wiley, New York, 1962).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
GABEL, N. Abiotic Formation of Phosphoric Anhydride Bonds in Dilute Aqueous Conditions. Nature 218, 354 (1968). https://doi.org/10.1038/218354a0
Received:
Revised:
Issue date:
DOI: https://doi.org/10.1038/218354a0