Abstract
The Australian Leukaemia Study Group (ALSG) investigated whether G-CSF would accelerate haemopoietic recovery after induction treatment for acute myeloid leukaemia (AML) intensified with high-dose cytarabine, and therefore improve response rates and survival. Patients were randomised to receive lenograstim (glycosylated recombinant human G-CSF) 5 μg per kg body weight subcutaneously daily from day 8 after starting chemotherapy, or no cytokine, following chemotherapy with cytarabine 3 g/m2 every 12 h on days 1, 3, 5, and 7, together with idarubicin 9 or 12 mg/m2 on days 1, 2, and 3, plus etoposide 75 mg/m2 on days 1 to 7 inclusive. Patients had untreated AML, and were aged 16 to 60 years. Overall, 54 evaluable patients were randomised to receive lenograstim and 58 to no cytokine. Patients in the lenograstim arm had a significantly shorter duration of neutropenia <0.5 × 109/l compared to patients in the no cytokine arm (median 18 vs 22 days; P = 0.0005), and also shorter duration of total leucopenia <1.0 × 109/l (17 vs 19 days; P = 0.0002), as well as a reduction in duration of treatment with therapeutic intravenous antibiotics (20 vs 24 days; P = 0.015) and a trend to reduced number of days with fever >38.0°C (9 vs 12 days; P = 0.18). There were no differences between the two groups in platelet recovery, red cell or platelet transfusions, or non-haematological toxicities. For patients achieving CR after their first induction course, a reduction in the time to the start of the next course of therapy was observed in the lenograstim arm, from a median of 40.5 days to a median of 36 days (P = 0.082). The overall complete response rates to chemotherapy were similar, 81% in the lenograstim arm vs 75% for the no cytokine arm (P = 0.5), and there was no significant difference in the survival durations. We conclude that the granulopoietic stimulating effect of G-CSF is observed after induction therapy for AML intensified by high-dose cytarabine, resulting in an improvement in a number of clinically important parameters with no major adverse effects.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bishop JF, Lowenthal RM, Joshua D, Matthews JP, Todd D, Cobcroft R, Whiteside M, Kronenberg H, Ma D, Dodds A, Herrmann R, Szer J, Wolf MM, Young G . Etoposide in acute non-lymphocytic leukaemia (ANLL) Blood 1990 75: 1–6
Bishop J, Matthews J, Young G, Szer J, Gillett A, Joshua D, Bradstock K, Enno A, Wolf M, Fox R, Cobcroft R, Herrmann R, van Der Weyden M, Lowenthal RM, Page F, Garson OM, Juneja S . A randomized study of high-dose cytarabine in induction in acute myeloid leukaemia Blood 1996 87: 1710–1717
Gabrilove JL, Jakubowski A, Scher H, Sternberg C, Wong G, Grous J, Yagoda A, Fain K, Moore MAS, Clarkson B, Oettgen HF, Alton K, Welte K, Souza L . Effect of granulocyte colony-stimulating factor on neutropenia and associated morbidity due to chemotherapy for transitional-cell carcinoma of the urothelium N Engl J Med 1988 318: 1414–1422
Crawford J, Ozer H, Stoller R, Johnson D, Lyman G, Tabbara I, Kris M, Grous J, Picozzi V, Rausch G, Smith R, Gradishar W, Yahanda A, Vincent M, Stewart M, Glaspy J . Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer N Engl J Med 1991 325: 164–170
Lowenberg B, Touw IP . Hematopoietic growth factors and their receptors in acute leukemia Blood 1993 81: 281–292
Ohno R, Tomonaga M, Kobayashi T, Kanamuru A, Shirakawa S, Masaoka T, Omine M, Oh H, Nomura T, Sakai Y, Hirano M, Yokomaku S, Nakayama S, Yoshida Y, Miura AB, Morishima Y, Dohy H, Niho Y, Hamajima N, Takaku F . Effect of granulocyte colony-stimulating factor after intensive induction therapy in relapsed or refractory acute leukemia N Engl J Med 1990 323: 871–877
Ohno R, Naoe T, Kanamaru A, Yoshida M, Hiraoka A, Kobayashi T, Ueda T, Minami S, Morishima Y, Saito Y, Furusawa S, Imai K, Takemoto Y, Miura Y, Teshima H, Hamajima N and the Kohseisho Leukemia Study Group . A double-blind controlled study of granulocyte colony-stimulating factor started two days before induction chemotherapy in refractory acute myeloid leukemia Blood 1994 83: 2086–2092
Buchner T, Hiddemann, W, Wormann B, Rottmann R, Zuhlsdorf M, Maschmeyer G, Ludwig WD, Sauerland MC, Frisch J, Schulz G . GM-CSF multiple course priming and long-term administration in newly diagnosed AML. Hematopoietic and therapeutic effects Blood 1994 84: (Suppl.1) Abstr. 27a
Heil G, Chadid L, Hoelzer D, Seipelt G, Mitrou P, Huber C, Kolbe K, Mertelsmann R, Lindemann A, Frisch J, Nicolay U, Gaus W, Heimpel H . GM-CSF in a double-blind randomized, placebo controlled trial in therapy of adult patients with de novo acute myeloid leukemia (AML) Leukemia 1995 5: 3–9
Rowe JM, Andersen J, Mazza JJ, Bennett JM, Paietta E, Hayes A, Oette D, Cassileth PA, Stadtmauer EA, Wiernik PH . A randomized placebo-controlled phase III study of granulocyte–macrophage colony-stimulating factor in adult patients (>55–70 years of age) with acute myelogenous leukemia. A study of the Eastern Cooperative Oncology Group (ECOG) Blood 1995 86: 457–462
Witz F, Harousseau JL, Sadoun A, Guyotat D, Berthou C, Cahn JY, Gardin C, Lioure B, Witz B, Desablens B, Ifrah N, Pignon B, Leprise PY, Audhuy B, Caillot D, Casassus P, Linassier P, Christian B, Hurteloup P, Polin V, Tellier Z . GM-CSF during and after remission induction treatment for elderly patients with acute myeloid leukemia (AML) Blood 1995 86: (Suppl 1.) Abstr. 512a
Lowenberg B, Boogaerts MA, Vellenga E, Ossenkoppele GJ, Dekker AW, van der Lelie J, Schouten HC, Fopp M, Gratwohl A, Fey M, Gmur J, van Putten WJL . Various modalities of use of GM-CSF in the treatment of acute myelogenous leukemia (AML). A Hovon-sakk randomized study (HOVON-4A) Blood 1995 86: (Suppl. 1) Abstr. 512a
Lowenberg B, Suciu S, Zittoun R, Ossenkoppele GJ, Boogaerts M, Wijermans PW, Vellenga E, Berneman ZN, Dekker AW, Sonneveld P, Stryckmans P, Solbu G, Dardenne M, de Witte T, Archimbaud E . GM-CSF during as well as after induction chemotherapy (CT) in elderly patients with acute myeloid leukemia (AML). The EORTC-HOVON Phase III Trial (AML 11) Blood 1995 86: (Suppl. 1) Abstr. 433a
Stone RM, Berg DT, George SL, Dodge RK, Paciucci PA, Schulman P, Lee EJ, Moore JO, Powell BL, Schiffer CA . Granulocyte–macrophage colony-stimulating factor after initial chemotherapy for elderly patients with primary acute myelogenous leukemia. Cancer and Leukemia Group B N Engl J Med 1995 332: 1671–1677
Godwin JE, Kopecky K, Head DR, Hynes HE, Balcerzak SP, Appelbaum FR . A double-blind placebo controlled trial of G-CSF in elderly patients with previously untreated acute myeloid leukemia. A Southwest Oncology Group Study Blood 1995 86: (Suppl. 1) Abstr. 434a
Dombret H, Chastang C, Fenaux P, Reiffers J, Bordessoule D, Bouabdallah R, Mandelli F, Ferrant A, Auzanneau G, Tilly H, Yver A, Degos L . A controlled study of recombinant human granulocyte colony-stimulating factor in elderly patients after treatment for acute myelogenous leukemia N Engl J Med 1995 332: 1678–1683
Zittoun R, Suciu S, Mandelli F, de Witte T, Thaler J, Stryckmans P, Hayat M, Peetermans M, Cadiou M, Solbu G, Petti MC, Willemze R . Granulocyte–macrophage colony-stimulating factor associated with induction treatment of acute myelogenous leukemia: a randomized trial by the European Organization for Research and Treatment of Cancer Leukemia Cooperative Group J Clin Oncol 1996 14: 2150–2159
Heil G, Hoelzer D, Sanz MA, Lechner K, Yin JAL, Papa G, Noens L, Szer J, Ganser A, O'Brien C, Matcham J, Barge A for the International Acute Myeloid Leukemia Study Group . A randomized, double-blind, placebo controlled, phase III study of filgrastim in remission induction and consolidation therapy for adults with de novo acute myeloid leukemia Blood 1997 90: 4710–4718
Wei LJ, Lachin JM . Properties of the URN randomization in clinical trials Control Clin Trials 1988 9: 345–364
Whitehead J . The Design and Analysis of Sequential Clinical Trials, (revised second edn) John Wiley: Chichester 1997
Brunier H, Whitehead J . PEST Planning and Evaluation of Sequential Trials, version 3, operating manual, 1993
Estey E, Thall P, Andreeff M, Beran M, Kantarjian H, O'Brien S, Escudier S, Robertson LE, Koller C, Kornblau S, Pierce S, Freireich EJ, Deisseroth A, Keating M . Use of granulocyte colony-stimulating factor before, during, and after fludarabine plus cytarabine induction therapy of newly diagnosed acute myelogenous leukemia or myelodysplastic syndromes: comparison with fludarabine plus cytarabine without granulocyte colony-stimulating factor J Clin Oncol 1994 12: 671–678
Bennett CL, Stinson TJ, Laver JH, Bishop MR, Godwin JE, Tallman MS . Cost analyses of adjunct colony stimulating factors for acute leukemia: can they improve clinical decision making Leuk Lymphoma 2000 37: 65–70
Lu ZJ, Luo R, Erder H, Heil G, Ganser A, Lechner K, Yin JL, Szer J, Barge A . Cost impact of filgrastim as an adjunct to chemotherapy for patients with acute myeloid leukemia Blood 1996 88: (Suppl. 1) 209a
Bennett CL, Hynes D, Godwin J, Stinson TJ, Golub RM, Apelbaum FR . Economic analysis of granulocyte colony-stimulating factor (G-CSF) as adjunct therapy for older patients with acute myelogenous leukemia (AML): estimates from a Southwest Oncology Group (SWOG) clinical trial Blood 1998 92: (Suppl. 1) 615a
Bennett CL, Stinson TJ, Tallman MS, Stadtmauer EA, Marsh RW, Friedenberg W, Lazarus HM, Kaminer L, Golub RM, Rowe JM for the Eastern Cooperative Oncology Group (E1490) . Economic analysis of a randomized placebo-controlled phase III study of granulocyte–macrophage colony-stimulating factor in adult patients (>55 to 70 years of age) with acute myelogenous leukemia Ann Oncol 1999 10: 177–182
Levin L, Hyrniuk W . Dose intensity analysis of chemotherapy regimens in ovarian carcinoma J Clin Oncol 1987 5: 756–767
Murray N . The importance of dose and dose intensity in lung cancer chemotherapy Semin Oncol 1987 14: (Suppl. 4) 20–28
Acknowledgements
This work was supported by research grant RG950971 from the National Health and Medical Research Council of Australia, by Pharmacia and Upjohn Australia, and by AMRAD Pharmaceuticals Pty Ltd, who provided lenograstim for this study. We gratefully acknowledge the contributions of the numerous data managers who provided information from contributing hospitals. We particularly acknowledge the assistance of the members of the independent safety monitoring committee (John Simes, Richard Fisher and Max Whiteside) who oversaw the conduct of this study. The following members of the Australian Leukaemia Study Group also contributed patients into this study: G Dart, J Ho, N Horvath, Royal Adelaide Hospital, Adelaide; P Elliott, Alfred Hospital, Melbourne; I Prosser, M Webb, Canberra Hospital, Canberra; F Cordingley, Fremantle Hospital, Perth; R Kimber, Royal Hobart Hospital, Hobart; J Gallo, P Motum, D Rosenfeld, Liverpool Hospital, Sydney; S Deveridge, M Seldon, A Spencer, Newcastle Mater Hospital, Newcastle; M Wolf, Peter MacCallum Cancer Institute, Melbourne; P Marlton, Princess Alexandra Hospital, Brisbane; P Bardy, J Szer, Royal Melbourne Hospital, Melbourne; D Ma, Royal North Shore Hospital, Sydney; R Baker, R Herrmann, Royal Perth Hospital, Perth; J Gibson, H Kronenberg, Royal Prince Alfred Hospital, Sydney; I Bunce, Wesley Medical Centre, Brisbane; P Castaldi, M Hertzberg, Westmead Hospital, Sydney. We are also grateful to Andrew Dalton, health economist, for invaluable advice on methods for resource analysis.
Author information
Authors and Affiliations
Consortia
Rights and permissions
About this article
Cite this article
Bradstock, K., Matthews, J., Young, G. et al. Effects of glycosylated recombinant human granulocyte colony-stimulating factor after high-dose cytarabine-based induction chemotherapy for adult acute myeloid leukaemia. Leukemia 15, 1331–1338 (2001). https://doi.org/10.1038/sj.leu.2402218
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sj.leu.2402218
Keywords
This article is cited by
-
Effectiveness and safety of primary prophylaxis with G-CSF after induction therapy for acute myeloid leukemia: a systematic review and meta-analysis of the clinical practice guidelines for the use of G-CSF 2022 from the Japan society of clinical oncology
International Journal of Clinical Oncology (2024)
-
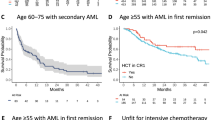
Dissecting causes for improved survival among patients with acute myeloid leukemia in two different eras receiving identical regimens in sequential randomized studies
Blood Cancer Journal (2018)
-
Myeloid growth factors in acute myeloid leukemia: systematic review of randomized controlled trials
Annals of Hematology (2011)
-
Role of cytokines in the treatment of acute leukemias: a review
Leukemia (2006)
-
Long-term survival data from a phase 3 study of Filgrastim as an adjunct to chemotherapy in adults with de novo acute myeloid leukemia
Leukemia (2006)